Abstract
Posttraumatic stress disorder (PTSD) has been shown to be associated with pro-inflammatory markers, including elevated plasma levels of interleukin-1β (IL-1β). However, the precise role of neuroinflammation and central immune signaling on the development of this debilitating psychological disorder is not known. Here, we used stress-enhanced fear learning (SEFL), an animal model of the disorder, to examine the role of central IL-1β in PTSD. The results show that the severe stressor in SEFL induces a time-dependent increase in IL-1β immunoreactivity and mRNA expression within the dentate gyrus of the dorsal hippocampus (DH). There was no increase in IL-1β in the basolateral amygdala or the perirhinal cortex. Moreover, blocking the action of IL-1β following the severe stressor with IL-1 receptor antagonist (10 μg, intracerebroventricular (i.c.v.), 24 and 48 h after the stressor) prevented the development of SEFL. To provide further support for the role of IL-1β in the development of SEFL, we show that systemic morphine, a treatment which is known to reduce both PTSD and SEFL, also reduces IL-1β expression in the DH induced by the severe stressor. These studies provide the first evidence that IL-1 is involved SEFL and suggest that IL-1 signaling in the brain may have a critical role in the development of PTSD.
Similar content being viewed by others
Introduction
Psychopathology and disease states involving depression and anxiety have been associated with pro-inflammatory markers in both human populations and preclinical rodent models (Spivak et al, 1997; Gill et al, 2009; Stepanichev et al, 2014). However, the precise role of neuroinflammation and immune signaling in this context remains unclear. Cytokines, traditionally known for their role in the periphery in defense against infection and disease, have recently been revealed as important signaling molecules in neuroglial communication pathways. For example, transgenic mouse lines deficient in central signaling involving pro-inflammtaory cytokines, such as interleukin-1β (IL-1β) or tumor necrosis factor-α (TNF-α), consistently exhibit altered anxiety behavior in the elevated plus maze or open-field test. (Silverman et al, 2007; Koo and Duman, 2009). Further, an infusion of IL-1β into the lateral ventricle is sufficient to induce anxiety-like behavior in the elevated plus maze (Koo and Duman, 2009). Thus, the immune system has the capability to modulate central signaling and play an important role in behavior and the development of psychopathology.
IL-1β has been implicated in a wide range of behaviors. Early studies implicated this cytokine in a specific behavioral complex, sickness behavior, which is characterized by sleep disorders, anxiety, and diminished social interactions (Dantzer, 2001). Importantly, IL-1β signaling is also critical for normal learning and memory processing. Accumulating evidence suggests that although a basal level of IL-1β is required for memory formation, both excessive and insufficient amounts of IL-1β impair memory formation (Goshen et al, 2007). Further, converging evidence suggests that many of the neurobiological parallels between a disease state and a state of stress, both of which are often characterized by sickness behavior-like phenotypes, may be explained by IL-1β signaling (Maier, 2003). There is evidence that IL-1β is upregulated throughout the brain after stress exposure (Nguyen et al, 1998; O’Connor et al, 2003) and is important in stress-induced hypothalamic–pituitary–adrenal axis regulatory responses (Goshen et al, 2008).
The overall goal of the present study was to examine the role of brain IL-1β in the development of stress-enhanced fear learning (SEFL), an animal model of posttraumatic stress disorder (PTSD) developed by Fanselow and colleagues (Rau et al, 2005). In PTSD, a severe traumatic event leads to debilitating psychological and physiological consequences characterized by chronic or exaggerated fear and anxiety (Gill et al, 2009). PTSD affects 7.7 million Americans (NIMH), including up to 17% of combat veterans (Richardson et al, 2010). However, there is no consistently effective treatment. Although several animal models of PTSD have been developed (van der Kolk, 1987; Yamamoto et al, 2009; Kaouane et al, 2012), the SELF paradigm is an outstanding model that captures critical components of human PTSD, including hyperarousal and hypersensitivity to future fear learning (Rau et al, 2005). In the SEFL paradigm, rats previously exposed to a severe stressor show an enhanced or exaggerated learned fear response to a later mild form of stress in a distinct context, a hallmark symptom of PTSD.
The present studies test the hypothesis that the severe stressor in SEFL is capable of inducing alterations in IL-1β expression in the brain and that this expression is functionally relevant to the development of enhanced fear learning. In Experiment 1, we examined the time course of IL-1β expression following the severe stressor in SEFL. Our analysis focused on the dorsal hippocampus (DH, including the dentate gyrus (DG), CA1, and CA3), basolateral amygdala (BLA), and perirhinal cortex (PrhC)—three regions that have been shown to be critical to fear conditioning (Anagnostaras et al, 2001; Kim et al, 2011; Acheson et al, 2012; Kent and Brown, 2012). In Experiment 2, we tested whether blocking central IL-1β signaling with an infusion of interleukin-1 receptor antagonist (IL-1ra) in the 48 h following the severe stressor prevented the development of SEFL. In Experiment 3, we tested whether morphine administration attenuated the induction of IL-1β following the severe stressor in SEFL. This third experiment is based upon prior studies in our laboratory using this model showing that morphine administered after the severe stressor is effective in preventing the development of SEFL (Szczytkowski-Thomson et al, 2013). Moreover, clinical studies also show that morphine administration in the hours after a combat injury or single-event trauma requiring hospitalization was associated with a reduced risk of PTSD (Bryant et al, 2009; Stoddard et al, 2009; Holbrook et al, 2010; Nixon et al, 2010; Melcer et al, 2014). Thus, in this third experiment, we hypothesize that IL-1β signaling is altered by morphine and therefore may be one mechanism through which morphine alters SEFL.
Materials and Methods
Animals
Male Sprague–Dawley rats (250–400 g, Charles River Laboratories, Raleigh, NC) were housed individually under a reversed light–dark (12-hour) cycle with ad libitum access to food and water. Animals were handled regularly during a 1-week acclimation period prior to experimentation. All procedures were conducted in accordance with and approval by the UNC Institutional Animal Care and Use Committee.
Experiment 1—Analysis of IL-1β Immunoreactivity and mRNA Expression Following the Severe Stressor in SEFL
Animals were exposed to only the initial stressor of our typical SEFL model. The SEFL model we used has been described previously (Szczytkowski-Thomson et al, 2013) and is based on the model of Fanselow and colleagues (Rau et al, 2005). In brief, in Experiments 1a and 1b, animals were placed into Context A for 90 min to receive 15 scrambled foot shocks (2 mA, 1 s) on a 6-min variable time schedule. Control animals were exposed to Context A for an equivalent amount of time without foot shocks.
In Experiment 1a, brain tissue was processed for immunohistochemical analysis. These animals (N=36, n=6) were assigned to either a no foot shock group (NS Control) or a foot shock group and one of five time points: 0, 6, 24, 48, or 72 h after the stressor. Animals in the NS Control group were killed 24 h after removal from Context A. This time point was chosen for our control group because any changes in IL-1β induced by learning from mere exposure to a novel context would be evident after 24 h (Goshen and Yirmiya, 2009). All other animals were exposed to Context A and killed via transcardial perfusion at the appropriate times.
In Experiment 1b, brain tissue was processed for quantitative polymerase chain reaction (qPCR) analysis of mRNA. These animals (N=32, n=8) were assigned to either a no foot shock group (NS Control) or a foot shock group and one of three time points, 0, 24, or 48 h after the stressor. Again, animals in the NS control group were killed 24 h after Context A exposure, and all other animals were killed via cervical dislocation at the appropriate times.
Experiment 2—Effect of a Central Infusion of IL-1ra on the Development of SEFL
Surgical procedures
Animals were surgically implanted with intracerebroventricular (i.c.v.) cannulae. Animals were anesthetized with a 1.0 mg/kg intraperitoneal injection of 9 : 1 (vol:vol) ketamine hydrochloride (100 mg/ml) mixed with xylazine (100 mg/ml). Guide cannulae (26 Gauge, Plastics One, Roanoke, VA) were directed at the right lateral ventricle (AP −0.9 mm, ML −1.5 mm, DV −3.4 mm, relative to bregma). Animals were given 3 weeks for postoperative recovery. Correct placements were verified and animals with incorrect placement were dropped from the analysis.
Procedures
Animals (N=36, n=9) were assigned to a Context A treatment (foot shock or no foot shock) and a drug treatment (IL-1ra or vehicle) and exposed to the SEFL paradigm. The first day of the SEFL paradigm is the foot shock treatment in Context A, as described in Experiment 1. Context A (BRS/LVE, Laurel, MD; 26.7 × 24.8 × 30.7) and Context B (Med Associates, St Albans, VT) were housed in separate rooms and had distinct textile, olfactory, and auditory characteristics, as described previously (Szczytkowski-Thomson et al, 2013). Context B was set-up to record the animals’ behavior using a video-recording system (Sony Video Camera Model HDR-CX150). Seven days after Context A exposure, animals were placed into Context B for 30 min. On day 8, animals were placed into Context B for a single scrambled foot shock (1 mA, 1 s) at 3 min, 12 s. On days 9, 10, 15, and 23 (test days 1, 2, 7 and 14), animals were placed in Context B for 8 min, 32 s and behavior was recorded to measure freezing behavior, a measure of learned fear, defined as a lack of all movement except that required for breathing. No animals in any group demonstrated freezing behavior to Context B during habituation/prior to the single foot shock, suggesting that there was no generalization of fear between contexts. Thus, any differences observed between treatment groups would reflect altered learning to the single foot shock in Context B. Videos were analyzed by raters blind to treatment condition.
IL-1ra (GenScript, Piscataway, NJ) was reconstituted in sterile saline (2.5 μg/μl). Twenty-four and 48 h after removal from Context A, animals were microinfused with 4 μl of either IL-1ra (10 μg) or sterile saline vehicle at a rate of 2 μl/min. Injectors were left in place for 4 min to allow for diffusion. These time points were based on our earlier finding that morphine administration prevents the development of SEFL when administered 48 h after, but not immediately after, Context A (Szczytkowski-Thomson et al, 2013).
Experiment 3—Analysis of IL-1β Immunoreactivity Following the Severe Stressor of SEFL in Combination with Morphine Treatment
Animals (N=28, n=7) were assigned to a Context A treatment (no foot shock or foot shock) and to a morphine treatment (morphine or vehicle). As described in Experiment 1, animals were only exposed to the initial stressor of the SEFL paradigm. Morphine was obtained from the National Institute on Drug Abuse and dissolved in sterile saline (7.5 mg/ml). Immediately, 24 and 48 h after removal from Context A, animals were administered either 7.5 mg/kg morphine or saline vehicle subcutaneously. One hour after the final injection, animals were killed via transcardial perfusion.
Immunohistochemical Analysis
In Experiments 1a and 3, animals were deeply anesthetized with 9 : 1 (vol:vol) ketamine hydrochloride (100 mg/ml), mixed with xylazine (100 mg/ml), and transcardially perfused with cold phosphate buffer (PB; pH=7.4) followed by 4% paraformaldehyde in 0.1 M PB. Brains were placed in 30% sucrose for cryoprotection and sliced into 40 μm sections. Tissue sections were washed for 15 min with 0.1 M PB, and incubated for 30 min at 80 °C in sodium citrate (pH=8.5) for antigen retrieval. Subsequently, sections were incubated for 30 min with streptavidin and biotin blocks (Vector Laboratories, Burlingame, CA) and pre-incubated for 60 min with 3% normal goat serum and 0.3% Triton X-100 in 0.1 M PB. Sections were incubated overnight with rabbit anti-IL-1β (1 : 1000; Abcam, Cambridge, MA) in 0.1 M PB with 3% normal goat serum and 0.3% Tween20. Similar to Johnson and Kan (2010), sections were then incubated for 60 min with biotinylated goat anti-rabbit IgG (1 : 1000, Vector Laboratories) in 1% normal goat serum and a streptavidin- Alexafluor488-conjugated tertiary antibody (1 : 1000, Life Technologies, Grand Island, NY) was used for visualization. Tissue sections labeled with only secondary and tertiary antibodies were used as secondary controls to ensure specificity of our primary antibody. Sections were mounted onto SuperFrost Plus slides (Fisher Scientific, Pittsburgh, PA) using Vectashield with DAPI hardset mounting medium (Vector Laboratories).
Color images were captured through a digital camera (Roper Scientific), mounted on an optical microscope (BX-51, Olympus), and positive fluorescence was quantified using ImageJ (NIH). Images captured were between −2.76 mm and −4.2 mm relative to bregma (Paxinos and Watson, 2007). Three to six sections were analyzed per animal for each brain region and values were averaged and expressed as positive stain per 5 μm2. Images were normalized to background fluorescence using a manual thresholding procedure such that the binary overlay completely covered all positive stain (similar to Sugama et al, 2011). In addition, the number of IL-1β-positive cells in the all images taken from the DG of the DH was counted manually. Images with high background that resulted from poor perfusion were dropped from the analysis. This decision was made at the time of thresholding and was made blind to the treatment condition. All thresholding and counting was conducted blind to the treatment condition. Publication images were compiled with Adobe Photoshop CS software. Color levels and background were reduced for optimal representation with levels and curves tools. Images from all experimental groups were treated equally.
qPCR Analysis
Following killing, brains were extracted and the hippocampus was immediately dissected out on a cold plate. The dorsal third of the hippocampus was stored in 5 × (vol/wt) RNA later (Qiagen) and immediately flash frozen. Tissue was processed by the UNC Animal Clinical Chemistry and Gene Expression Laboratories according to protocols previously reported (Kim et al, 2002). In brief, brain tissue was homogenized in RNA lysis buffer (PE Biosystems, Foster City, CA) with Ca2 and Mg2- free PB using a Fast Prep 120 mixer (QBIOgene, Vista, CA). RNA isolations were purified using the ABI Prism 6700 automated nucleic-acid workstation (PE Biosystems) according to the manufacturer’s protocol. Real-time RT-PCR reactions were performed in the ABI Prism 7700 sequence detector (PE Biosystems) in a total volume of 30 μl (10 μl RNA, 20 μl reaction mixture). Each qPCR amplification was performed in duplicate: 30 min at 48 °C for the RT reaction and 10 min at 94 °C followed by 40 temperature cycles (15 s at 94 °C and 1 min at 60 °C). Signal intensity was normalized to β-actin as an endogenous control. The nucleotide sequences of the PCR primers and fluorogenic probes used for the IL-1β and β-actin genes were as follows: IL-1β forward: 5′-GCCTCAAGGGGAAGAATCTA-3′, reverse: 5′-ATCCACACTCTCCAGCTGC-3′, probe: 5′-FCTGTGTAATGAAAGACGGCACACCCACQ-3′; β-actin forward: 5′-TGCCTGACGGTCAGGTCA-3′, reverse: 5′-CAGGAAGGAAGGCTGGAAG-3′, probe: 5′-FCACTATCGGCAATGAGCGGTTCCGQ-3′.
Statistical Analysis
For Experiments 1a and 1b, positive fluorescence and cell counts were analyzed using a one-way ANOVA with treatment group as the between-subjects factor. For Experiment 2, a 2 × 2 ANOVA with Context A treatment and drug treatment as between-subjects factors was used to analyze baseline-freezing behavior. A 2 × 2 × 4 ANOVA with Context A treatment and drug treatment as between-subjects factors and test day as a within-subjects factor was used to analyze freezing behavior across test days. At last, for Experiment 3, positive fluorescence and cell counts were analyzed using a 2 × 2 ANOVA with Context A treatment and morphine treatment as between-subjects factors. Significant interactions were examined using Fisher’s least significant difference (LSD) post hoc tests.
Results
Experiment 1: Severe Stressor Enhances IL-1β Immunoreactivity and mRNA Expression in the DH but not BLA or PrhC
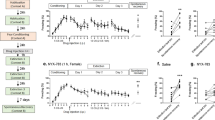
In Experiment 1a, IL-1β immunoreactivity was significantly enhanced by exposure to the severe stressor in a time-sensitive manner such that an increase was observed beginning 6 h after the severe stressor and persisted through 72 h (Figure 1). Severe stress significantly enhanced IL-1β immunoreactivity in the DG both in terms of the percent area of positive fluorescence, F (5, 25)=6.472, p<0.01, and in terms of IL-1β-positive cell counts, F (5, 16)=13.544, p<0.01. LSD post hoc tests revealed that compared with the no foot shock group, IL-1β expression was significantly enhanced at 6, 24, 48, and 72 h (p<0.01). In contrast, IL-1β expression in CA1, CA3, the BLA, and the PrhC was not altered by the severe stressor at any of the time points (Figure 1).
Severe stress induced IL-1β in the dorsal hippocampus. Representative images ( × 20) of IL-1β (green) in the dorsal hippocampus from animals in each of the six groups are shown on the left. The top right panel shows ImageJ analysis of positive fluorescence stain (top right) in the dentate gyrus, CA1, CA3, basolateral amygdala, and perirhinal cortex. IL-1β immunoreactivity was significantly enhanced at 6 h after (but not immediately after) severe stress only in the dentate gyrus of the dorsal hippocampus. This enhancement persisted through 72 h (*p<0.05 compared with NS control). qPCR analysis of mRNA expression confirmed the IL-1β increase in the dorsal hippocampus that we observed with immunohistochemistry (bottom right). IL-1β mRNA was enhanced at 48 h following severe stress (*p<0.05 compared with NS control). Further, IL-1β-positive cells were also counted in the dentate gyrus of the dorsal hippocampus (bottom right) and the same pattern was confirmed. (*p<0.05 compared with 0 h). Error bars indicate SEM.
In Experiment 1b, exposure to the severe stressor significantly enhanced IL-1β mRNA expression in the DH in a time-dependent manner, F (3, 26)=6.927, p<0.001 (Figure 1). LSD post hoc tests revealed that, compared with the no foot shock group, IL-1β mRNA expression was significantly enhanced at 48 h (p<0.01). Although mRNA and protein reflect distinct points of the IL-1 signal, the discrepancy in the time course of the effect between Experiments 1a and 1b may reflect the observation in Experiment 1 that the effect is specific to the DG. The tissue processed for qPCR included whole-DH samples, and thus the time course of the effect may be different from the immunohistochemical analysis of the DH, as the CA1/3 did not achieve significance. Still, Experiment 1b further confirms an increase in IL-1β on a delayed timeline in the DH following foot shock.
Experiment 2: IL-1ra Prevents the Development of SEFL
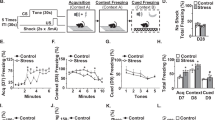
There was no effect of Context A treatment or drug treatment on baseline freezing in Context B, indicating no generalization of fear between contexts, F (3, 38)=1.91, p>0.05. A 2 × 2 × 4 ANOVA revealed a significant main effect of Context A treatment, F (1, 36)=11.08, p<0.01, and a significant main effect of drug treatment, F (1, 36)=12.99, p<0.01. There was also a significant effect of test day, indicating that conditioned fear diminished over time, F (3, 34)=21.76, p<0.01. Most interestingly, there was a significant Context A treatment by drug treatment interaction, F (1, 36)=6.497, p<0.05. LSD post hoc comparisons showed that the animals that received foot shock followed by vehicle exhibited significantly more freezing behavior compared with animals that received no foot shock in Context A followed by vehicle (p<0.01), confirming a significant SEFL effect. The animals that received foot shock in Context A followed by IL-1ra and the animals that received no foot shock in Context A followed by IL-1ra did not differ in freezing behavior exhibited in Context B, p>0.05. Thus, these results show that SEFL was prevented by an i.c.v. administration of IL-1ra (Figure 2).
IL-1ra prevents the development of SEFL. IL-1ra infusion (i.c.v., 10 μg at 24 and 48 h after removal from Context A) significantly reduced conditioned freezing behavior in Context B across test days. There were no significant differences between groups at baseline. Within the vehicle-treated groups, foot shock in Context A (open squares) significantly enhanced freezing behavior in Context B compared with animals that did not receive foot shock in Context A (open circles), demonstrating an SEFL effect. There was no effect of IL-1ra in the group that did not receive foot shock in Context A (closed circles). Most importantly, animals that received foot shock in Context A followed by IL-1ra infusion (closed squares) did not differ from the groups that did not receive foot shock in Context A. Error bars indicate SEM.
Experiment 3: Morphine Administration Attenuates Severe Stressor-Induced IL-1β Immunoreactivity in the DH
Severe stress significantly enhanced IL-1β expression within vehicle-treated animals in the DG of the DH, and this enhancement was significantly attenuated in animals that received foot shock followed by the morphine treatment (Figure 3). A 2 × 2 ANOVA revealed a main effect of Context A treatment, F (1, 19)=78.164, p<0.001, and a main effect of morphine treatment, F (1, 19)=13.651, p<0.01. Importantly, there was a significant Context A treatment by morphine treatment interaction, F (1, 19)=31.050, p<0.01. LSD post hoc comparisons revealed that animals that received foot shock in Context A followed by vehicle treatment exhibited significantly enhanced IL-1β expression compared with animals that received no foot shock in Context A followed by vehicle treatment (p<0.001), replicating the effect observed in Experiment 1a. Animals that received no foot shock in Context A followed by morphine treatment were not significantly different from animals that received no foot shock in Context A followed by vehicle. However, animals that received foot shock in Context A followed by the morphine treatment exhibited significantly less IL-1β expression compared with the group that received foot shock in Context A followed by vehicle, p<0.001. Similar to Experiment 1a, all statistical analyses were confirmed by the data obtained from counts of IL-1β-positive cells (Figure 3).
Morphine attenuates stress-induced IL-1β in the dorsal hippocampus. Representative images ( × 20) of IL-1β (green) in the dentate gyrus of the dorsal hippocampus from animals in each of the four groups are shown in (a). ImageJ analysis of positive fluorescence stain (b) revealed that in vehicle treated animals (NS/Veh and Shock/Veh), foot shock significantly enhanced IL-1β; however, morphine administration suppressed the induction of IL-1β in animals that received foot shock followed by morphine treatment (Shock/M). IL-1β- positive cells were also counted in the dentate gyrus of the dorsal hippocampus (c) and the same pattern was confirmed. (*greater than NS/Veh, p<0.05; †greater than Shock/M, p<0.05). Error bars indicate SEM.
Discussion
The current studies demonstrate for the first time that exposure to a severe stressor that is capable of producing a PTSD-like phenotype in rats induces a time-dependent increase in IL-1β in the DH. Furthermore, central administration of IL-1ra prevents subsequent effects of that stressor, namely, the development of SEFL. Previously, our laboratory established that morphine following the severe stressor also blocks the development of SEFL (Szczytkowski-Thomson et al, 2013). Here, we show that the same systemic morphine treatment also attenuates stress-induced IL-1β expression in the DH. These studies suggest that morphine may act through an IL-1β-dependent mechanism to alter SEFL. Collectively, the experiments provide new evidence that the expression of IL-1β in the brain following a severe stressor is a critical component of the development of SEFL, a model of PTSD.
In the present study, IL-1β was detected in three brain regions known to be important in PTSD and fear acquisition and expression—the hippocampus, amygdala, and PrhC (Kim et al, 2011; Acheson et al, 2012). These structures are extensively interconnected with multiple pathways, involving bidirectional synaptic plasticity (Kent and Brown, 2012). However, the effect of foot shock on IL-1β levels was observed only in the DH, with no change in immunoreactivity observed in the BLA or PrhC. More specifically, within the DH, the effect was most pronounced in the DG with much less expression that was not statistically altered by foot shock in CA1 and CA3. There may be other areas yet to be identified that also have an important role. For example, the ventral hippocampus is thought to be important in the emotional and affective component of memory (Fanselow and Dong, 2010) but it is not known what role IL-1 might have in the ventral hippocampus.
Several cell types are capable of producing IL-1β, and both neurons and glia express IL-1 receptors (Yabuuchi et al, 1994; Zhang et al, 2010). In astrocytes, IL-1β initiates the formation of IL-1β-signaling intermediates, such as IL-1 receptor-associated kinase (Ringwood and Li, 2008; Flannery and Bowie, 2010) and the subsequent activation of p38, extracellular signal-regulated kinase (ERK) and nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB; (Guasch et al, 2007; Huang et al, 2011)). Conversely, in hippocampal neurons, IL-1β activates p38 and ERK, but not NFkB (Huang et al, 2011). It should also be acknowledged that IL-1ra blocks multiple forms of IL-1, including IL-1α as well as IL-1β. The relative contributions of these different forms of IL-1 should be considered when determining the cellular sources of IL-1.
The mechanism through which IL-1β induces alterations in SEFL is not known. There are studies showing that neuroinflammation modulates synaptic plasticity in the hippocampus by influencing long-term potentiation (LTP), a process thought to underlie some forms of learning. For example, TNF-α concentrations have been found to influence LTP in a dose-dependent manner such that extremely high TNF-α concentrations severely inhibited LTP in hippocampal neurons (Tancredi et al, 1992). Furthermore, Schneider and colleagues found that tetanic stimulation used to induce LTP induced an increase in IL-1β mRNA expression specifically in hippocampal slices (Schneider et al, 1998) and that IL-1ra impaired the maintenance of LTP. Similarly, IL-1 receptor knockout mice did not exhibit LTP in the DG (Avital et al, 2003). Thus, there are a number of studies indicating that the cytokines are involved in both short- and long-term changes in synaptic plasticity.
Physiological evidence that cytokines influence synaptic plasticity is also supported by behavioral evidence that central cytokine signaling influences learning in several paradigms, including fear conditioning (Yirmiya and Goshen, 2011; Szczytkowski et al, 2013). For example, acute administration of either TNF-α or IL-1β and chronic administration of interleukin-6 (IL-6) enhanced memory performance in a passive avoidance paradigm (Matsuda et al, 1996; Yirmiya et al, 2002; Brennan et al, 2004). Furthermore, there is evidence for an effect of IL-1ra in step-down passive avoidance learning, but some report memory improvement (Depino et al, 2004), whereas others have reported memory impairment(Yirmiya et al, 2002). Early studies have also shown that morphine administration, which we show here to reduce hippocampal IL-1β, reduces memory for step-down avoidance(Izquierdo, 1979). Thus, it is clear that IL-1β can alter learning and memory, but the direction of the effects may depend upon such factors as the timing of the administration, the severity of stressor, and the type of learning being examined. The present findings are unique in that they begin to address the role of IL-1β in the neural plastic changes required to alter future learning, ie, SEFL.
The present findings suggest that key neurobiological changes that render the animal hypersensitive to future fear learning occur on a delayed timeline, in concert with the timeline of some forms of memory consolidation. In support, Kozlovsky et al (2012) found a similarly time-sensitive effect in the predator-scent stress paradigm, another animal model of PTSD (Kozlovsky et al, 2012). An administration of corticotrophin-releasing hormone receptor antisense oligodeoxynucleotide into the DH 48 h, but not immediately, after severe stress significantly reduced the prevalence of the extreme behavioral response in the elevated plus maze and the acoustic startle response test, which is thought to reflect PTSD-like behavior (Kozlovsky et al, 2012). This is consistent with prior data from our laboratory showing that the 48 h time point is the key (Szczytkowski-Thomson et al, 2013). Interestingly, the effect of morphine on the prognosis of PTSD in combat veterans was also time-dependent such that the relationship between morphine administration and a reduced likelihood of PTSD was strongest when it was administered in level 2 care, which occurs not immediately on the battlefield but within 72 h of the initial trauma (Melcer et al, 2014). Frankland and Bontempi suggested a model of memory consolidation in which components are integrated in the hippocampus into a single-memory trace which, over time, becomes consolidated to cortical structures and integrated with other experiences (termed systems consolidation) (Frankland and Bontempi, 2005). One hypothesis is that SEFL (and PTSD) is (are) driven by an alteration in the later phases of memory consolidation, following the trauma that results in the loss of contextual detail and renders the animal hypersensitive to future fear learning.
The present studies do not test whether morphine or IL-1ra impair contextual fear learning to Context A. We cannot assume that manipulations that attenuate SEFL, such as IL-1ra or morphine, also impair memory for Context A. Fanselow and colleagues (2005) showed that contextual fear learning to the context where the initial severe shock occurs is not critical to the expression of SEFL. They found that eliminating fear learning to Context A and extinction of Context A did not affect the expression of SEFL in Context B (Rau et al, 2005). Taken together, it would be interesting to know how blocking IL-1 influences fear learning to Context A and further whether IL-1β expression is directly involved in the learning/enhanced learning to the subsequent foot shock in Context B.
PTSD is a prevalent problem in the United States with severe fiscal and emotional costs to society. The current findings provide strong evidence that the correlation between psychopathology and inflammatory dysregulation (eg, (Bai et al, 2014; Stepanichev et al, 2014) is important in behavioral outcomes. Moreover, Melcer and colleagues found that susceptibility to PTSD was related to traumatic brain injury severity in combat veterans (Melcer et al, 2014), further suggesting that central inflammation is related to the development of PTSD. The current studies show that altering central immune signaling has a direct causal effect on the development of a PTSD-like phenotype in rats. Further, we provide evidence that morphine, a treatment known to reduce the development of PTSD and SEFL, may act through an IL-1β-dependent mechanism, providing an exciting new target for the development of treatments for PTSD.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
References
Acheson DT, Gresack JE, Risbrough VB (2012). Hippocampal dysfunction effects on context memory: possible etiology for posttraumatic stress disorder. Neuropharmacology 62: 674–685.
Anagnostaras SG, Gale GD, Fanselow MS (2001). Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus 11: 8–17.
Avital A, Goshen I, Kamsler A, Segal M, Iverfeldt K, Richter-Levin G et al (2003). Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus 13: 826–834.
Bai YM, Su TP, Tsai SJ, Wen-Fei C, Li CT, Pei-Chi T et al (2014). Comparison of inflammatory cytokine levels among type I/type II and manic/hypomanic/euthymic/depressive states of bipolar disorder. J Affect Disord 166: 187–192.
Brennan FX, Beck KD, Servatius RJ (2004). Proinflammatory cytokines differentially affect leverpress avoidance acquisition in rats. Behav Brain Res 153: 351–355.
Bryant RA, Creamer M, O'Donnell M, Silove D, McFarlane AC (2009). A study of the protective function of acute morphine administration on subsequent posttraumatic stress disorder. Biol Psychiatry 65: 438–440.
Dantzer R (2001). Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun 15: 7–24.
Depino AM, Alonso M, Ferrari C, del Rey A, Anthony D, Besedovsky H et al (2004). Learning modulation by endogenous hippocampal IL-1: blockade of endogenous IL-1 facilitates memory formation. Hippocampus 14: 526–535.
Fanselow MS, Dong HW (2010). Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65: 7–19.
Flannery S, Bowie AG (2010). The interleukin-1 receptor-associated kinases: Critical regulators of innate immune signalling. Biochem Pharmacol 80: 1981–1991.
Frankland PW, Bontempi B (2005). The organization of recent and remote memories. Nat Rev Neurosci 6: 119–130.
Gill JM, Saligan L, Woods S, Page G (2009). PTSD is associated with an excess of inflammatory immune activities. Perspect Psychiatr Care 45: 262–277.
Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T et al (2008). Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry 13: 717–728.
Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T et al (2007). A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology 32: 1106–1115.
Goshen I, Yirmiya R (2009). Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol 30: 30–45.
Guasch RM, Blanco AM, Pérez-Aragó A, Miñambres R, Talens-Visconti R, Peris B et al (2007). RhoE participates in the stimulation of the inflammatory response induced by ethanol in astrocytes. Exp Cell Res 313: 3779–3788.
Holbrook TL, Galarneau MR, Dye JL, Quinn K, Dougherty AL (2010). Morphine use after combat injury in Iraq and post-traumatic stress disorder. N Engl J Med 362: 110–117.
Huang Y, Smith DE, Ibáñez-Sandoval O, Sims JE, Friedman WJ (2011). Neuron-specific effects of interleukin-1beta are mediated by a novel isoform of the IL-1 receptor accessory protein. J Neurosci 31: 18048–18059.
Izquierdo I (1979). Effect of naloxone and morphine on various forms of memory in the rat: possible role of engogenous opiate mechanisms in memory consolidation. Psychopharmacology (Berl) 66: 199–203.
Johnson EA, Kan RK (2010). The acute phase response and soman-induced status epilepticus: temporal, regional and cellular changes in rat brain cytokine concentrations. J Neuroinflammation 7: 40.
Kaouane N, Porte Y, Vallée M, Brayda-Bruno L, Mons N, Calandreau L et al (2012). Glucocorticoids can induce PTSD-like memory impairments in mice. Science 335: 1510–1513.
Kent BA, Brown TH (2012). Dual functions of perirhinal cortex in fear conditioning. Hippocampus 22: 2068–2079.
Kim HS, Lee G, John SW, Maeda N, Smithies O (2002). Molecular phenotyping for analyzing subtle genetic effects in mice: application to an angiotensinogen gene titration. Proc Natl Acad Sci USA 99: 4602–4607.
Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN et al (2011). The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res 223: 403–410.
Koo JW, Duman RS (2009). Interleukin-1 receptor null mutant mice show decreased anxiety-like behavior and enhanced fear memory. Neurosci Lett 456: 39–43.
Kozlovsky N, Zohar J, Kaplan Z, Cohen H (2012). Microinfusion of a corticotrophin-releasing hormone receptor 1 antisense oligodeoxynucleotide into the dorsal hippocampus attenuates stress responses at specific times after stress exposure. J Neuroendocrinol 24: 489–503.
Maier SF (2003). Bi-directional immune-brain communication: Implications for understanding stress, pain, and cognition. Brain Behav Immun 17: 69–85.
Matsuda S, Wen TC, Morita F, Otsuka H, Igase K, Yoshimura H et al (1996). Interleukin-6 prevents ischemia-induced learning disability and neuronal and synaptic loss in gerbils. Neurosci Lett 204: 109–112.
Melcer T, Walker J, Sechriest VF 2nd, Lebedda M, Quinn K, Galarneau M (2014). Glasgow Coma Scores, early opioids, and posttraumatic stress disorder among combat amputees. J Trauma Stress 27: 152–159.
Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR et al (1998). Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci 18: 2239–2246.
Nixon RD, Nehmy TJ, Ellis AA, Ball SA, Menne A, McKinnon AC (2010). Predictors of posttraumatic stress in children following injury: The influence of appraisals, heart rate, and morphine use. Behav Res Ther 48: 810–815.
OConnor KA, Johnson JD, Hansen MK, Wieseler Frank JL, Maksimova E, Watkins LR et al (2003). Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res 991: 123–132.
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates 6th edn Academic Press: San Diego.
Rau V, DeCola JP, Fanselow MS (2005). Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev 29: 1207–1223.
Richardson LK, Frueh BC, Acierno R (2010). Prevalence estimates of combat-related post-traumatic stress disorder: critical review. Aust N Z J Psychiatry 44: 4–19.
Ringwood L, Li L (2008). The involvement of the interleukin-1 receptor-associated kinases (IRAKs) in cellular signaling networks controlling inflammation. Cytokine 42: 1–7.
Schneider H, Pitossi F, Balschun D, Wagner A, del Rey A, Besedovsky HO (1998). A neuromodulatory role of interleukin-1beta in the hippocampus. Proc Natl Acad Sci USA 95: 7778–7783.
Silverman MN, Macdougall MG, Hu F, Pace TW, Raison CL, Miller AH (2007). Endogenous glucocorticoids protect against TNF-alpha-induced increases in anxiety-like behavior in virally infected mice. Mol Psychiatry 12: 408–417.
Spivak B, Shohat B, Mester R, Avraham S, Gil-Ad I, Bleich A et al (1997). Elevated levels of serum interleukin-1 beta in combat-related posttraumatic stress disorder. Biol Psychiatry 42: 345–348.
Stepanichev M, Dygalo NN, Grigoryan G, Shishkina GT, Gulyaeva N (2014). Rodent models of depression: neurotrophic and neuroinflammatory biomarkers. Biomed Res Int 2014: 932757.
Stoddard FJ Jr, Sorrentino EA, Ceranoglu TA, Saxe G, Murphy JM, Drake JE et al (2009). Preliminary evidence for the effects of morphine on posttraumatic stress disorder symptoms in one- to four-year-olds with burns. J Burn Care Res 30: 836–843.
Sugama S, Takenouchi T, Sekiyama K, Kitani H, Hashimoto M (2011). Immunological responses of astroglia in the rat brain under acute stress: interleukin 1 beta co-localized in astroglia. Neuroscience 192: 429–437.
Szczytkowski JL, Lebonville C, Hutson L, Fuchs RA, Lysle DT (2013). Heroin-induced conditioned immunomodulation requires expression of IL-1beta in the dorsal hippocampus. Brain Behav Immun 30: 95–102.
Szczytkowski-Thomson JL, Lebonville CL, Lysle DT (2013). Morphine prevents the development of stress-enhanced fear learning. Pharmacol Biochem Behav 103: 672–677.
Tancredi V, DArcangelo G, Grassi F, Tarroni P, Palmieri G, Santoni A et al (1992). Tumor necrosis factor alters synaptic transmission in rat hippocampal slices. Neurosci Lett 146: 176–178.
van der Kolk BA (1987). The drug treatment of post-traumatic stress disorder. J Affect Disord 13: 203–213.
Yabuuchi K, Minami M, Katsumata S, Satoh M (1994). Localization of type-I interleukin-1 receptor messenger-Rna in the rat-brain. Mol Brain Res 27: 27–36.
Yamamoto S, Morinobu S, Takei S, Fuchikami M, Matsuki A, Yamawaki S et al (2009). single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress Anxiety 26: 1110–1117.
Yirmiya R, Goshen I (2011). Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 25: 181–213.
Yirmiya R, Winocur G, Goshen I (2002). Brain interleukin-1 is involved in spatial memory and passive avoidance conditioning. Neurobiol Learn Mem 78: 379–389.
Zhang R, Sun L, Hayashi Y, Liu X, Koyama S, Wu Z et al (2010). Acute p38-mediated inhibition of NMDA-induced outward currents in hippocampal CA1 neurons by interleukin-1beta. Neurobiol Dis 38: 68–77.
Acknowledgements
Dr Lysle’s work has been funded by the NIH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jones, M., Lebonville, C., Barrus, D. et al. The Role of Brain Interleukin-1 in Stress-Enhanced Fear Learning. Neuropsychopharmacol 40, 1289–1296 (2015). https://doi.org/10.1038/npp.2014.317
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2014.317
This article is cited by
-
Dorsal hippocampal interleukin-1 signaling mediates heroin withdrawal-enhanced fear learning
Psychopharmacology (2020)
-
Repeated social defeat-induced neuroinflammation, anxiety-like behavior and resistance to fear extinction were attenuated by the cannabinoid receptor agonist WIN55,212-2
Neuropsychopharmacology (2018)
-
Modeling a linkage between blood transcriptional expression and activity in brain regions to infer the phenotype of schizophrenia patients
npj Schizophrenia (2017)
-
Glial and Neuroimmune Mechanisms as Critical Modulators of Drug Use and Abuse
Neuropsychopharmacology (2017)
-
Therapeutic Implications of Brain–Immune Interactions: Treatment in Translation
Neuropsychopharmacology (2017)