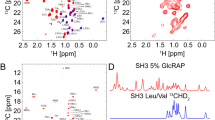
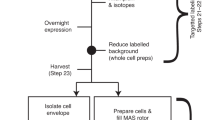
Abstract
The development of isotope labeling methodology has had a significant impact on NMR studies of high-molecular-weight proteins and macromolecular complexes. Here we review some of this methodology that has been developed and used in our laboratory. In particular, experimental protocols are described for the production of highly deuterated, uniformly 15N- and 13C-labeled samples of large proteins, with optional incorporation of selective isotope labels into methyl groups of isoleucine, leucine and valine residues. Various types of methyl labeling schemes are assessed, and the utility of different methyl labeling strategies is highlighted for studies ranging from protein structure determination to the investigation of side-chain dynamics. In the case of malate synthase G (MSG), the time frame of the whole preparation, including the protein refolding step, is about 70 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tugarinov, V., Hwang, P.M. & Kay, L.E. Nuclear magnetic resonance spectroscopy of high-molecular-weight proteins. Annu. Rev. Biochem. 73, 107–146 (2004).
Wider, G. & Wüthrich, K. NMR spectroscopy of large molecules and multimolecular assemblies in solution. Curr. Opin. Struct. Biol. 9, 594–601 (1999).
Pervushin, K., Riek, R., Wider, G. & Wüthrich, K. Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc. Natl. Acad. Sci. USA 94, 12366–12371 (1997).
Rosen, M.K. et al. Selective methyl group protonation of perdeuterated proteins. J. Mol. Biol. 263, 627–636 (1996).
Gardner, K.H., Zhang, X., Gehring, K. & Kay, L.E. Solution NMR studies of a 42 kDa E. coli maltose binding protein/β cyclodextrin complex: chemical shift assignments and analysis. J. Am. Chem. Soc. 120, 11738–11748 (1998).
Tugarinov, V., Muhandiram, R., Ayed, A. & Kay, L.E. Four-dimensional NMR spectroscopy of a 723-residue protein: chemical shift assignments and secondary structure of malate synthase G. J. Am. Chem. Soc. 124, 10025–10035 (2002).
Tugarinov, V. & Kay, L.E. Ile, Leu, and Val methyl assignments of the 723-residue malate synthase G using a new labeling strategy and novel NMR methods. J. Am. Chem. Soc. 125, 13868–13878 (2003).
Rajesh, S. et al. A novel method for the biosynthesis of deuterated proteins with selective protonation at the aromatic rings of Phe, Tyr and Trp. J. Biomol. NMR 27, 81–86 (2003).
Coughlin, P.E. et al. Improved resolution and sensitivity of triple-resonance NMR methods for the structural analysis of proteins by use of a backbone-labeling strategy. J. Am. Chem. Soc. 121, 11871–11874 (1999).
Metzler, W.J., Wittekind, M., Goldfarb, V., Mueller, L. & Farmer, B.T. Incorporation of 1H/13C/15N-{Ile,Leu,Val} into a perdeuterated, 15N-labeled protein: potential in structure determination of large proteins by NMR. J. Am. Chem. Soc. 118, 6800–6801 (1996).
Grzesiek, S., Anglister, J., Ren, H. & Bax, A. 13C line narrowing by 2H decoupling in 2H/13C/15N-enriched proteins. Applications to triple resonance 4D J-connectivity of sequential amides. J. Am. Chem. Soc 115, 4369–4370 (1993).
Kainosho, M. et al. Optimal isotope labelling for NMR protein structure determinations. Nature 440, 52–57 (2006).
Janin, J., Miller, S. & Chothia, C. Surface, subunit interfaces and interior of oligomeric proteins. J. Mol. Biol. 204, 155–164 (1988).
Tugarinov, V. & Kay, L.E. Methyl groups as probes of structure and dynamics in NMR studies of high-molecular-weight proteins. Chembiochem 6, 1567–1577 (2005).
Gardner, K.H. & Kay, L.E. Production and incorporation of 15N, 13C, 2H (1H-δ1 methyl) isoleucine into proteins for multidimensional NMR studies. J. Am. Chem. Soc. 119, 7599–7600 (1997).
Goto, N.K., Gardner, K.H., Mueller, G.A., Willis, R.C. & Kay, L.E. A robust and cost-effective method for the production of Val, Leu, Ile (δ1) methyl-protonated 15N-,13C-,2H-labeled proteins. J. Biomol. NMR 13, 369–374 (1999).
Tugarinov, V. & Kay, L.E. An isotope labeling strategy for methyl TROSY spectroscopy. J. Biomol. NMR 28, 165–172 (2004).
Tugarinov, V. & Kay, L.E. Stereospecific NMR assignments of prochiral methyls, rotameric states and dynamics of valine residues in malate synthase G. J. Am. Chem. Soc. 126, 9827–9836 (2004).
Tugarinov, V., Choy, W.Y., Orekhov, V.Y. & Kay, L.E. Solution NMR-derived global fold of a monomeric 82-kDa enzyme. Proc. Natl. Acad. Sci. USA 102, 622–627 (2005).
Tugarinov, V., Hwang, P.M., Ollerenshaw, J.E. & Kay, L.E. Cross-correlated relaxation enhanced 1H-13C NMR spectroscopy of methyl groups in very high molecular weight proteins and protein complexes. J. Am. Chem. Soc. 125, 10420–10428 (2003).
Korzhnev, D.M., Kloiber, K., Kanelis, V., Tugarinov, V. & Kay, L.E. Probing slow dynamics in high molecular weight proteins by methyl-TROSY NMR spectroscopy: application to a 723-residue enzyme. J. Am. Chem. Soc. 126, 3964–3973 (2004).
Tugarinov, V., Ollerenshaw, J.E. & Kay, L.E. Probing sidechain dynamics in high molecular weight proteins by deuterium NMR spin relaxation: an application to an 82-kDa enzyme. J. Am. Chem. Soc. 127, 8214–8225 (2005).
Tugarinov, V. & Kay, L.E. Quantitative 13C and 2H NMR relaxation studies of the 723-residue enzyme malate synthase G reveal a dynamic binding interface. Biochemistry 44, 15970–15977 (2005).
Millet, O., Muhandiram, D.R., Skrynnikov, N.R. & Kay, L.E. Deuterium spin probes of sidechain dynamics in proteins. 1. Measurement of five relaxation rates per deuteron in 13C-labeled and fractionally 2H-enriched proteins in solution. J. Am. Chem. Soc. 124, 6439–6448 (2002).
Howard, B.R., Endrizzi, J.A. & Remington, S.J. Crystal structure of Escherichia coli malate synthase G complexed with magnesium and glyoxylate at 2.0 Å resolution: mechanistic implications. Biochemistry 39, 3156–3168 (2000).
McCaldon, P. & Argos, P. Oligopeptide biases in protein sequences and their use in predicting protein coding regions in nucleotide sequences. Proteins 4, 99–122 (1988).
Ollerenshaw, J.E., Tugarinov, V., Skrynnikov, N.R. & Kay, L.E. Comparison of 13CH3, 13CH2D, and 13CHD2 methyl labeling strategies in proteins. J. Biomol. NMR 33, 25–41 (2005).
Acknowledgements
The authors gratefully acknowledge many of the former members of the Kay laboratory who have contributed to the development of the selective methyl protonation approaches described here. V.T. is a recipient of a Canadian Institutes of Health Research Postdoctoral Fellowship. L.E.K. is the recipient of a Canada Research Chair in Biochemistry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Tugarinov, V., Kanelis, V. & Kay, L. Isotope labeling strategies for the study of high-molecular-weight proteins by solution NMR spectroscopy. Nat Protoc 1, 749–754 (2006). https://doi.org/10.1038/nprot.2006.101
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.101
This article is cited by
-
A squarate-pillared titanium oxide quantum sieve towards practical hydrogen isotope separation
Nature Communications (2023)
-
Combined NMR and molecular dynamics conformational filter identifies unambiguously dynamic ensembles of Dengue protease NS2B/NS3pro
Communications Biology (2023)
-
Mechanism of antibody-specific deglycosylation and immune evasion by Streptococcal IgG-specific endoglycosidases
Nature Communications (2023)
-
Three segment ligation of a 104 kDa multi-domain protein by SrtA and OaAEP1
Journal of Biomolecular NMR (2023)
-
Distinct dissociation rates of murine and human norovirus P-domain dimers suggest a role of dimer stability in virus-host interactions
Communications Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.