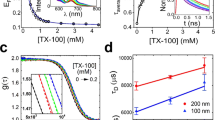
Abstract
The solubilization and reconstitution of biological or liposomal membranes by detergents and biomolecules with detergent-like properties play a major role for technical applications (e.g., the isolation of membrane proteins) and biological phenomena (of, e.g., amphiphilic peptides). It is therefore important to know and understand the amounts of a given detergent required for the onset and completion of membrane solubilization and the detergent–lipid interactions in general. Lipid–detergent systems can form a variety of aggregate structures, which can be grouped into two pseudophases (lamellae and micelles) so that solubilization can be approximately described as a phase transition. Here we present a protocol for establishing the phase diagram and a detailed thermodynamic description of a lipid–detergent system based on isothermal titration calorimetry (ITC). The protocol can also be used to detect additive-induced membrane destabilization, permeabilization, domain formation and lipid-dependent transitions between rod-like and spherical micelles. A minimal protocol consisting of all sample preparation procedures and a single solubilization experiment can be accomplished within 2 days; a more extensive series comprising both solubilization and reconstitution experiments requires several days to a few weeks, depending on the number of titrations performed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
le Maire, M., Champeil, P. & Møller, J.V. Interaction of membrane proteins and lipids with solubilizing detergents. Biochim. Biophys. Acta 1508, 86–111 (2000).
Ollivon, M., Lesieur, S., Grabielle-Madelmont, C. & Paternostre, M. Vesicle reconstitution from lipid–detergent mixed micelles. Biochim. Biophys. Acta 1508, 34–50 (2000).
Heerklotz, H. Interactions of surfactants with lipid membranes. Q. Rev. Biophys. 41, 205–264 (2008).
Helenius, A. & Simons, K. Solubilization of membranes by detergents. Biochim. Biophys. Acta 415, 29–79 (1975).
Lasch, J. Interaction of detergents with lipid vesicles. Biochim. Biophys. Acta 1241, 269–292 (1995).
Lichtenberg, D., Opatowski, E. & Kozlov, M.M. Phase boundaries in mixtures of membrane-forming amphiphiles and micelle-forming amphiphiles. Biochim. Biophys. Acta 1508, 1–19 (2000).
Lichtenberg, D. Characterization of the solubilization of lipid bilayers by surfactants. Biochim. Biophys. Acta 821, 470–478 (1985).
Cladera, J., Rigaud, J.L., Villaverde, J. & Dunach, M. Liposome solubilization and membrane protein reconstitution using Chaps and Chapso. Eur. J. Biochem. 243, 798–804 (1997).
Rigaud, J.L., Pitard, B. & Levy, D. Reconstitution of membrane proteins into liposomes: application to energy-transducing membrane proteins. Biochim. Biophys. Acta 1231, 223–246 (1995).
Geertsma, E.R., Mahmood, N.A.B.N., Schuurman-Wolters, G.K. & Poolman, B. Membrane reconstitution of ABC transporters and assays of translocator function. Nat. Protoc. 3, 256–266 (2008).
Tsamaloukas, A.D., Keller, S. & Heerklotz, H. Uptake and release protocol for assessing membrane binding and permeation by way of isothermal titration calorimetry. Nat. Protoc. 2, 695–704 (2007).
Heerklotz, H., Lantzsch, G., Binder, H., Klose, G. & Blume, A. Application of isothermal titration calorimetry for detecting lipid membrane solubilization. Chem. Phys. Lett. 235, 517–520 (1995).
Heerklotz, H., Lantzsch, G., Binder, H., Klose, G. & Blume, A. Thermodynamic characterization of dilute aqueous lipid/detergent mixtures of POPC and C12EO8 by means of isothermal titration calorimetry. J. Phys. Chem. 100, 6764–6774 (1996).
Heerklotz, H. Membrane stress and permeabilization induced by asymmetric incorporation of compounds. Biophys. J. 81, 184–195 (2001).
Heerklotz, H.H., Binder, H. & Schmiedel, H. Excess enthalpies of mixing in phospholipid–additive membranes. J. Phys. Chem. B. 102, 5363–5368 (1998).
Heerklotz, H., Szadkowska, H., Anderson, T. & Seelig, J. The sensitivity of lipid domains to small perturbations demonstrated by the effect of triton. J. Mol. Biol. 329, 793–799 (2003).
Garidel, P., Hildebrand, A., Knauf, K. & Blume, A. Membranolytic activity of bile salts: influence of biological membrane properties and composition. Molecules 12, 2292–2326 (2007).
Goñi, F.M. & Alonso, A. Spectroscopic techniques in the study of membrane solubilization, reconstitution and permeabilization by detergents. Biochim. Biophys. Acta 1508, 51–68 (2000).
Edwards, K. & Almgren, M. Solubilization of lecithin vesicles by C12E8 . J. Coll. Interf. Sci. 147, 1–21 (1991).
Walter, A., Vinson, P.K., Kaplun, A. & Talmon, Y. Intermediate structures in the cholate–phosphatidylcholine vesicle micelle transition. Biophys. J. 60, 1315–1325 (1991).
Goñi, F.M. et al. The interaction of phosphatidylcholine bilayers with Triton X-100. Eur. J. Biochem. 160, 659–665 (1986).
Heerklotz, H. Triton promotes domain formation in lipid raft mixtures. Biophys. J. 83, 2693–2701 (2002).
Jackson, M.L., Schmidt, C.F., Lichtenberg, D., Litman, B.J. & Albert, A.D. Solubilization of phosphatidylcholine bilayers by octyl glucoside. Biochemistry 21, 4576–4582 (1982).
Ollivon, M., Eidelman, O., Blumenthal, R. & Walter, A. Micelle–vesicle transition of egg phosphatidylcholine and octyl glucoside. Biochemistry 27, 1695–1703 (1988).
Hjelm, R.P., Thiyagarajan, P. & Alkanonyuksel, H. Organization of phosphatidylcholine and bile-salt in rodlike mixed micelles. J. Phys. Chem. 96, 8653–8661 (1992).
Keller, S., Heerklotz, H., Jahnke, N. & Blume, A. Thermodynamics of lipid membrane solubilization by sodium dodecyl sulfate. Biophys. J. 90, 4509–4521 (2006).
Keller, M., Kerth, A. & Blume, A. Thermodynamics of interaction of octyl glucoside with phosphatidylcholine vesicles: partitioning and solubilization as studied by high sensitivity titration calorimetry. Biochim. Biophys. Acta 1326, 178–192 (1997).
Opatowski, E., Kozlov, M.M. & Lichtenberg, D. Partitioning of octyl glucoside between octyl glucoside/phosphatidylcholine mixed aggregates and aqueous media as studied by isothermal titration calorimetry. Biophys. J. 73, 1448–1457 (1997).
Roth, Y., Opatowski, E., Lichtenberg, D. & Kozlov, M.M. Phase behavior of dilute aqueous solutions of lipid–surfactant mixtures: effects of finite size of micelles. Langmuir 26, 2052–2061 (2000).
Chellani, M. Isothermal titration calorimetry: biological applications. Am. Biotechnol. Lab. 17, 14–18 (1999).
Hope, M.J., Bally, M.B., Webb, G. & Cullis, P.R. Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim. Biophys. Acta 812, 55–65 (1985).
MacDonald, R.C. et al. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim. Biophys. Acta 1061, 297–303 (1991).
Heerklotz, H., Binder, H., Lantzsch, G., Klose, G. & Blume, A. Lipid/detergent interaction thermodynamics as a function of molecular shape. J. Phys. Chem. B. 101, 639–645 (1997).
Opatowski, E., Lichtenberg, D. & Kozlov, M.M. The heat of transfer of lipid and surfactant from vesicles into micelles in mixtures of phospholipid and surfactant. Biophys. J. 73, 1458–1467 (1997).
Wenk, M.R. & Seelig, J. Vesicle–micelle transformation of phosphatidylcholine/octyl-β-D-glucopyranoside mixtures as detected with titration calorimetry. J. Phys. Chem. B. 101, 5224–5231 (1997).
Tan, A.M., Ziegler, A., Steinbauer, B. & Seelig, J. Thermodynamics of sodium dodecyl sulfate partitioning into lipid membranes. Biophys. J. 83, 1547–1556 (2002).
Hildebrand, A., Beyer, K., Neubert, R., Garidel, P. & Blume, A. Temperature dependence of the interaction of cholate and deoxycholate with fluid model membranes and their solubilization into mixed micelles. Colloids Surf. B. Biointerfaces 32, 335–351 (2003).
Hildebrand, A., Neubert, R., Garidel, P. & Blume, A. Bile salt induced solubilization of synthetic phosphatidylcholine vesicles studied by isothermal titration calorimetry. Langmuir 18, 2836–2847 (2002).
Epand, R.M. & Epand, R.F. Calorimetric detection of curvature strain in phospholipid bilayers. Biophys. J. 66, 1450–1456 (1994).
Heerklotz, H.H., Binder, H. & Schmiedel, H. Excess enthalpies of mixing in phospholipid–additive membranes. J. Phys. Chem. B. 102, 5363–5368 (1998).
Wenk, M.R. & Seelig, J. Magainin 2 amide interaction with lipid membranes: calorimetric detection of peptide binding and pore formation. Biochemistry 37, 3909–3916 (1998).
Wieprecht, T., Apostolov, O., Beyermann, M. & Seelig, J. Membrane binding and pore formation of the antibacterial peptide PGLa: thermodynamic and mechanistic aspects. Biochemistry 39, 442–452 (2000).
Ongena, M. & Jacques, P. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 16, 115–125 (2008).
Heerklotz, H. & Seelig, J. Leakage and lysis of lipid membranes induced by the lipopeptide surfactin. Eur. Biophys. J. 36, 305–314 (2007).
Binder, H. & Lindblom, G. Charge-dependent translocation of the Trojan peptide penetratin across lipid membranes. Biophys. J. 85, 982–995 (2003).
Bárány-Wallje, E. et al. A critical reassessment of penetratin translocation across lipid membranes. Biophys. J. 89, 2513–2521 (2005).
Sauer, I. et al. Dipalmitoylation of a cellular uptake-mediating apolipoprotein E-derived peptide as a promising modification for stable anchorage in liposomal drug carriers. Biochim. Biophys. Acta 1758, 552–561 (2006).
Keller, S. et al. Membrane-mimetic nanocarriers formed by a dipalmitoylated cell-penetrating peptide. Angew. Chem. Int. Ed. 44, 5252–5255 (2005).
Heerklotz, H.H., Binder, H. & Epand, R.M. A 'release' protocol for isothermal titration calorimetry. Biophys. J. 76, 2606–2613 (1999).
Keller, S., Heerklotz, H. & Blume, A. Monitoring lipid membrane translocation of sodium dodecyl sulfate by isothermal titration calorimetry. J. Am. Chem. Soc. 128, 1279–1286 (2006).
Keller, S., Tsamaloukas, A. & Heerklotz, H. A quantitative model describing the selective solubilization of membrane domains. J. Am. Chem. Soc. 127, 11469–11476 (2005).
Beck, A., Tsamaloukas, A., Jurcevic, P. & Heerklotz, H. Additive action of two or more solutes on lipid membranes. Langmuir 24, 8833–8840 (2008).
Acknowledgements
This study was supported by grant 341920-07 from NSERC to H.H. and by grant KE 1478/1-1 from the Deutsche Forschungsgemeinschaft (DFG) to S.K.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heerklotz, H., Tsamaloukas, A. & Keller, S. Monitoring detergent-mediated solubilization and reconstitution of lipid membranes by isothermal titration calorimetry. Nat Protoc 4, 686–697 (2009). https://doi.org/10.1038/nprot.2009.35
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.35
This article is cited by
-
Isothermal titration calorimetry
Nature Reviews Methods Primers (2023)
-
Unveiling the multi-step solubilization mechanism of sub-micron size vesicles by detergents
Scientific Reports (2019)
-
How different sterols contribute to saponin tolerant plasma membranes in sea cucumbers
Scientific Reports (2018)
-
Integration and global analysis of isothermal titration calorimetry data for studying macromolecular interactions
Nature Protocols (2016)
-
Modulating bilayer mechanical properties to promote the coupled folding and insertion of an integral membrane protein
European Biophysics Journal (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.