Abstract
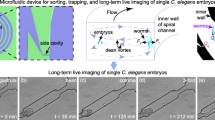
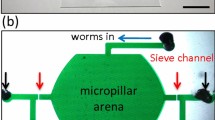
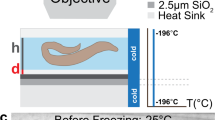
We present a protocol for building and operating a microfluidic device for mechanical immobilization of Caenorhabditis elegans in its physiologically active state. The system can be used for in vivo imaging of dynamic cellular processes such as cell division and migration, degeneration, aging and regeneration, as well as for laser microsurgery, Ca2+ imaging and three-dimensional microscopy. The device linearly orients C. elegans, and then completely restrains its motion by pressing a flexible membrane against the animal. This technique does not involve any potentially harmful anesthetics, gases or cooling procedures. The system can be installed on any microscope and operated using only one syringe and one external valve, making it accessible to most laboratories. The device fabrication begins by patterning photoresist structures on silicon wafers, which are then used to mold features in elastomeric layers that are thermally bonded to form the device. The system can be assembled within 3 d.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout













Similar content being viewed by others
References
Yanik, M.F. et al. Neurosurgery: functional regeneration after laser axotomy. Nature 432, 822–822 (2004).
Kerr, R., Lev-Ram, V., Baird, G., Vincent, P., Tsien, R.Y. & Schafer, W.R. Optical imaging of calcium transients in neurons and pharyngeal muscle of C. elegans. Neuron 26, 583–594 (2000).
Sulston, J. & Hodgkin, J. Methods. In The Nematode C. elegans (ed. Wood, W.) 587–606 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, USA, 1998).
Massie, M.R., Lapoczka, E.M., Boggs, K.D., Stine, K.E. & White, G.E. Exposure to the metabolic inhibitor sodium azide induces stress protein expression and thermotolerance in the nematode Caenorhabditis elegans. Cell Stress Chaperones 8, 1–7 (2003).
Podbilewicz, B. & Gruenbaum, Y. Live imaging of Caenorhabditis elegans. In Live Cell Imaging: A Laboratory Manual 1st edn. Chapter 20 (eds. Goldman, R.D. & Spector, D.L.) (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 2005).
Lewbart, G.A. & Bodri, M.S. Nematodes. In Invertebrate Medicine 1st edn. (ed. Lewbart, G.A.) (Blackwell Publishing, Ames, IA, 2006).
Rohde, C.B., Zeng, F., Gonzalez-Rubio, R., Angel, M. & Yanik, M.F. Microfluidic system for on-chip high-throughput whole-animal sorting and screening at subcellular resolution. Proc. Natl Acad. Sci. USA 104, 13891–13895 (2007).
Zeng, F., Rohde, C.B. & Yanik, M.F. Subcellular precision on-chip small-animal immobilization, multi-photon imaging and femtosecond laser manipulation. Lab Chip 8, 653–656 (2008).
Gray, J. et al. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature 430, 317–322 (2004).
Lange, D., Storment, C., Conley, C. & Kovacs, G. A microfluidic shadow imaging system for the study of the nematode C. elegans in space. Sens. Actuators B. 107, 904–914 (2005).
Heng, X. et al. Optofluidic microscopy—a method for implementing a high resolution optical microscope on a chip. Lab Chip 6, 1274–1276 (2006).
Qin, J. & Wheeler, A. Maze exploration and learning in C. elegans. Lab Chip 7, 186–192 (2007).
Chronis, N., Zimmer, M. & Bargmann, C. Microfluidics for in vivo imaging of neuronal and behavioral activity in C. elegans. Nat. Methods 4, 727–731 (2007).
Hulme, S.E., Shevkoplyas, S.S., Apfeld, J., Fontana, W. & Whitesides, G.M. A microfabricated array of clamps for immobilizing and imaging C. elegans. Lab Chip 7, 1515–1523 (2007).
Lockery, S. et al. Artificial dirt: microfluidic substrates for nematode neurobiology and behavior. J. Neurophysiol. 99, 3136–3143 (2008).
Chung, K., Crane, M.M. & Lu, H. Automated on-chip rapid microscopy, phenotyping and sorting of C. elegans. Nat. Methods 5, 637–643 (2008).
Guo, S. et al. Femtosecond laser nanoaxotomy lab-on-a-chip for in vivo nerve regeneration studies. Nat. Methods 5, 531–533 (2008).
Chokshi, T.V., Ben-Yakar, A. & Chronis, N. CO2 and compressive immobilization of C. elegans on-chip. Lab Chip 9, 151–157 (2009).
Yanik, M.F. et al. Nerve Regeneration in C. elegans after femtosecond laser axotomy. IEEE J. Sel. Top. Quantum Electron. 12, 1283–1291 (2006).
Steinmeyer, J. et al. Construction of a femtosecond laser microsurgery system. Nat. Protoc. 5, 395–407 (2010).
Unger, M.A., Chou, H.P., Thorsen, T., Scherer, A. & Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 288, 113–116 (2000).
Fang-Yen, C., Wasserman, S., Sengupta, P. & Samuel, A.D.T. Agarose immobilization of C. elegans. Worm Breed. Gaz. 18, 32 (2009).
Qin, D., Xia, Y. & Whitesides, G. Soft lithography and micro- and nanoscale patterning. Nat. Protoc. 5, 491–502 (2010).
Lee, J.N., Park, C. & Whitesides, G.M. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal. Chem. 75, 6544–6554 (2003).
Acknowledgements
We thank the following funding sources: NIH Director’s New Innovator Award Program (1-DP2-OD002989), Packard Award in Science and Engineering, Sloan Award in Neuroscience, NSF Career Award, NSF Graduate Research Fellowship, NSERC Fellowship and NIH Biotechnology Training Grant. We also thank S. Quake and the Stanford Microfluidics Foundry for advice regarding device fabrication.
Author information
Authors and Affiliations
Contributions
C.B.R. and F.Z. developed and characterized the microfluidic immobilization procedure. C.L.G. engineered the immobilization technique for manual operation, developed troubleshooting techniques and wrote the paper. C.L.G. and C.B.R. developed the other elements of the system. M.F.Y. supervised the project at all stages.
Corresponding author
Ethics declarations
Competing interests
The authors have filed patents on this technology. M.F.Y. is founder and chief scientific advisor of Entera Pharmaceuticals.
Supplementary information
Supplementary Figure 1
AutoCAD mask design file (ZIP 467 kb)
Supplementary Figure 2
PDF, AutoCAD mask design file, all layers (PDF 61 kb)
Supplementary Figure 3
PDF, AutoCAD mask design file, flow-1 layer (PDF 19 kb)
Supplementary Figure 4
PDF, AutoCAD mask design file, flow-2 layer (PDF 41 kb)
Supplementary Figure 5
PDF, AutoCAD mask design file, compress-1 layer (PDF 29 kb)
Supplementary Video
On-chip laser micro-surgery (MPG 436 kb)
Rights and permissions
About this article
Cite this article
Gilleland, C., Rohde, C., Zeng, F. et al. Microfluidic immobilization of physiologically active Caenorhabditis elegans. Nat Protoc 5, 1888–1902 (2010). https://doi.org/10.1038/nprot.2010.143
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2010.143
This article is cited by
-
Neuroscience Research using Small Animals on a Chip: From Nematodes to Zebrafish Larvae
BioChip Journal (2021)
-
Rotatable microfluidic device for simultaneous study of bilateral chemosensory neurons in Caenorhabditis elegans
Microfluidics and Nanofluidics (2020)
-
Microfluidic immobilization and subcellular imaging of developing Caenorhabditis elegans
Microfluidics and Nanofluidics (2017)
-
A microfluidic diode for sorting and immobilization of Caenorhabditis elegans
Biomedical Microdevices (2017)
-
Microbeam irradiation of C. elegans nematode in microfluidic channels
Radiation and Environmental Biophysics (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.