Abstract
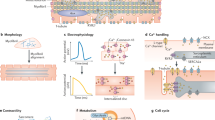
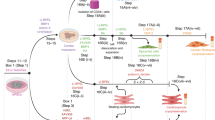
This protocol describes how to isolate primary cardiomyocytes from adult zebrafish hearts and culture them for up to 4 weeks, thereby using them as an alternative to in vivo experiments. After collagenase digestion of the ventricle, cells are exposed to increasing calcium concentrations in order to obtain high-purity cardiomyocytes. The whole isolation process can be accomplished in 4–5 h. The culture conditions we established allow the cells to preserve their mature sarcomeric integrity and contractile properties. Furthermore, adult zebrafish cardiomyocytes in culture, similarly to zebrafish in vivo heart regeneration, undergo partial dedifferentiation and, in contrast to their mammalian counterparts, are able to proliferate. Our protocol enables the study of structural and functional properties in close-to-native cardiomyocytes and allows the application of in vitro techniques and assays that are not feasible to perform in living animals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Major, R.J. & Poss, K.D. Zebrafish heart regeneration as a model for cardiac tissue repair. Drug Discov. Today Dis. Models 4, 219–225 (2007).
Kuhl, S.J. & Kuhl, M. Improving cardiac regeneration after injury: are we a step closer? Bioessays 33, 669–673 (2011).
Bersell, K., Arab, S., Haring, B. & Kuhn, B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 138, 257–270 (2009).
Engel, F.B. et al. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 19, 1175–1187 (2005).
Jopling, C. et al. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 464, 606–609 (2010).
Kikuchi, K. et al. Primary contribution to zebrafish heart regeneration by gata4+ cardiomyocytes. Nature 464, 601–605 (2010).
Gonzalez-Rosa, J.M. & Mercader, N. Cryoinjury as a myocardial infarction model for the study of cardiac regeneration in the zebrafish. Nat. Protoc. 7, 782–788 (2012).
Brette, F. et al. Characterization of isolated ventricular myocytes from adult zebrafish (Danio rerio). Biochem. Biophys. Res. Commun. 374, 143–146 (2008).
Zhang, P.C., Llach, A., Sheng, X.Y., Hove-Madsen, L. & Tibbits, G.F. Calcium handling in zebrafish ventricular myocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R56–R66 (2011).
Louch, W.E., Sheehan, K.A. & Wolska, B.M. Methods in cardiomyocyte isolation, culture, and gene transfer. J. Mol. Cell Cardiol. 51, 288–298 (2011).
Mitcheson, J.S., Hancox, J.C. & Levi, A.J. Cultured adult cardiac myocytes: future applications, culture methods, morphological and electrophysiological properties. Cardiovasc. Res. 39, 280–300 (1998).
Grunow, B. et al. In vitro developed spontaneously contracting cardiomyocytes from rainbow trout as a model system for human heart research. Cell Physiol. Biochem. 27, 1–12 (2011).
Nurmi, A. & Vornanen, M. Electrophysiological properties of rainbow trout cardiac myocytes in serum-free primary culture. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R1200–R1209 (2002).
Warren, K.S., Baker, K. & Fishman, M.C. The slow mo mutation reduces pacemaker current and heart rate in adult zebrafish. Am. J. Physiol. Heart Circ. Physiol. 281, H1711–H1719 (2001).
Jopling, C., Sune, G., Faucherre, A., Fabregat, C. & Izpisua Belmonte, J.C. Hypoxia induces myocardial regeneration in zebrafish. Circulation 126, 3017–3027 (2012).
Jopling, C., Suñe, G., Morera, C. & Izpisua Belmonte, J.C. p38α MAPK regulates myocardial regeneration in zebrafish. Cell Cycle 11, 1195–1201 (2012).
Frank, J.S., Rich, T.L., Beydler, S. & Kreman, M. Calcium depletion in rabbit myocardium. Ultrastructure of the sarcolemma and correlation with the calcium paradox. Circ. Res. 51, 117–130 (1982).
Singal, P.K., Matsukubo, M.P. & Dhalla, N.S. Calcium-related changes in the ultrastructure of mammalian myocardium. Br. J. Exp. Pathol. 60, 96–106 (1979).
Brand, N.J., Lara-Pezzi, E., Rosenthal, N. & Barton, P.J. Analysis of cardiac myocyte biology in transgenic mice: a protocol for preparation of neonatal mouse cardiac myocyte cultures. Methods Mol. Biol. 633, 113–124 (2010).
Nakagawa, O. et al. Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. Evidence for brain natriuretic peptide as an “emergency” cardiac hormone against ventricular overload. J. Clin. Invest. 96, 1280–1287 (1995).
Brutsaert, D.L. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol. Rev. 83, 59–115 (2003).
Kikuchi, K. et al. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev. Cell 20, 397–404 (2011).
Vieira, J.M. & Riley, P.R. Epicardium-derived cells: a new source of regenerative capacity. Heart 97, 15–19 (2011).
Kim, J., Rubin, N., Huang, Y., Tuan, T.L. & Lien, C.L. In vitro culture of epicardial cells from adult zebrafish heart on a fibrin matrix. Nat. Protoc. 7, 247–255 (2012).
Hedhli, N. et al. Endothelial-derived neuregulin is an important mediator of ischaemia-induced angiogenesis and arteriogenesis. Cardiovasc. Res. 93, 516–524 (2012).
Jopling, C., Boue, S. & Izpisua Belmonte, J.C. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat. Rev. Mol. Cell Biol. 12, 79–89 (2011).
Porrello, E.R. et al. Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080 (2011).
Huang, C.J., Tu, C.T., Hsiao, C.D., Hsieh, F.J. & Tsai, H.J. Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev. Dyn. 228, 30–40 (2003).
Poss, K.D., Wilson, L.G. & Keating, M.T. Heart regeneration in zebrafish. Science 298, 2188–2190 (2002).
McCurley, A.T. & Callard, G.V. Characterization of housekeeping genes in zebrafish: male-female differences and effects of tissue type, developmental stage and chemical treatment. BMC Mol. Biol. 9, 102 (2008).
Manoli, M. & Driever, W. Fluorescence-activated cell sorting (FACS) of fluorescently tagged cells from zebrafish larvae for RNA isolation. Cold Spring Harb. Protoc. http://dx.doi.org/10.1101/pdb.prot069633 (2012).
Kassen, S.C. et al. The Tg(ccnb1:EGFP) transgenic zebrafish line labels proliferating cells during retinal development and regeneration. Mol. Vis. 14, 951–963 (2008).
Dispersyn, G.D. et al. Dissociation of cardiomyocyte apoptosis and dedifferentiation in infarct border zones. Eur. Heart J. 23, 849–57 (2002).
Wang, J. et al. The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development 138, 3421–3430 (2011).
Acknowledgements
We thank A. Raya for help on the protocol, I. Berniakovich and C. Newey for critical reading of the manuscript and C. Fabregat, D. Mulero and J. Vaquero for technical assistance. This work was supported by an INSERM ATIP-AVENIR grant (C.J.). Work in the laboratory of J.C.I.B. was supported by TERCEL-ISCIII-MINECO, Fundacion Cellex, CIBER, The Leona M. and Harry B. Helmsley Charitable Trust and the G. Harold and Leila Y. Mathers Charitable Foundation.
Author information
Authors and Affiliations
Contributions
V.S., C.J. and J.C.I.B. designed the study; V.S., G.S., C.J. and C.M. carried out the experiments; and V.S. and J.C.I.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
Proliferation rate of primary cardiomyocytes. (a) Percentage of BrdU positive cardiomyocytes during the first week of culture. Cells were grown in the presence of 10 μM BrdU, fixed with 4% PFA at the indicated time points, and immunocytologically labelled for Tropomyosin and BrdU. Cells positive for Tropomyosin (as marker for cardiomyocytes), and BrdU were counted using the MetaMorph software. (b) Same experimental approach as in a, comparing the proliferation properties of cardiomyocytes obtained from control hearts to regenerating hearts (7 dpa), in presence and absence of serum. (PDF 258 kb)
Supplementary Figure 2
Viability decline of long term cultures. (a) Primary cardiomyocytes cultured for 5 weeks display a high amount of dead cells (separated, flattened cells, and round cells overlaying the living, network-forming cells; see also Supplementary Video 5). (b) Increase of percentage of dead cells (propidium iodide positive cells) after 10 days of culture. Scale bar, 100 μm. (PDF 302 kb)
Supplementary Figure 3
ANEP staining of cell-cell connections. (a,b) 10-days culture of primary cardiomyocytes. The cells were incubated in 10 μM di-3-ANEPPDHQ (Invitrogen, D36801, diluted in plating medium) for 15 min, washed, and imaged by confocal microscopy. The voltage-sensitive ANEP dye labels the action potential in the sarcolemma of the cardiomyocytes. See corresponding Supplementary Video 6 for contractile behaviour of this culture. Scale bars, 25 μm (a) and 10 μm (a'). (PDF 299 kb)
Supplementary Figure 4
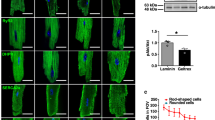
Cardiomyocyte marker expression in purified cells. (a) qPCR measuring gene expression of tissue specific markers, for the myocardium: cmlc2a; endocardium: fli1a; epicardium: wt1b and tcf21; cardiac fibroblasts or immature cardiomyocytes; vim. 18S ribosomal RNA was used for normalization. For the crude cell suspension (red bars), RNA was isolated from the cell pellet after the first wash (step 10), while for the sample of purified cardiomyocytes (green bars) the cell pellet obtained after all 7 washes (step 13) was used. Transcripts of all genes were detected in the crude cell suspension (Ct values displayed on y-axis), while only the normalizer and the cardiomyocyte marker cmlc2a were detectable in the purified sample. (b) Western blot showing a-SA (alpha sarcomeric actin) protein expression in samples obtained from whole zebrafish hearts (lane 1) and from purified cardiomyocytes (lane 2). Protein extracts prepared from purified cardiomyocytes are devoid of unspecific bands. (PDF 259 kb)
Zebrafish qPCR primers used: 18S ribosomal RNA: fwd, TCGCTAGTTGGCATCGTTTATG; rev, CGGAGGTTCGAAGACGATCA34. Cmlc2a (cardiac myosin light chain 2a also named myl7, myosin light polypeptide 7): fwd, GGCTCTTCCAATGTCTTCTCC; rev, GGACTCCAGCTCTTCATCAC. Fli1a (friend leukaemia integration 1a): fwd, CACCGAGGTCCTTCTCTCAC; rev, CTCTCCGTTGGTTCCTTCCC. Wt1b (wilms tumor 1b): fwd, CCACACAGAAATGCCAAATG; rev, GACCCAGCACATCTTGTC. Tcf21 (transcription factor 21): fwd, GTCCAGAGGAACGCTGCTAAC; rev, GTCAGGTTGACGGGATGTATG. Vim (vimentin): fwd, GGAAAAGAGCAAAGTGGAGGT; rev, GATCTGCATCTCAGCAAGTTC.
Supplementary Figure 5
Dedifferentiated cells originate from cardiomyocytes. (a) 7-days culture of primary cardiomyocytes displaying rod shaped cells as well as partially dedifferentiated, flattened cells (arrows). (b) mCherry under the control of the beta-actin promoter remains expressed in dedifferentiated cells, proving their myocardial origin. (c) Overlay of a and b. (d) 14-days culture of cardiomyocytes with advanced dedifferentiation and proliferation, immunocytologically labelled for Tropomyosin, Mef2c, BrdU, and counterstained with DAPI. The arrows point to cells with reduced expression of Tropomyosin, and strong expression of Mef2c, proving their myocardial identity4,33. (e-h) Nuclear DAPI staining overlaps with the Mef2c label in every cell, whereas some of the nuclei did not incorporate BrdU. Scale bars, 25 μm (a) and 100 μm (d). Lineage tracing was performed after crossing tg:cmlc2a-Ert2CreErt2 to tg:(eab2:[EGFP-T-mCherry]) zebrafish5. Driven by the beta-actin promoter, the F1 fish are ubiquitously GFP positive. Upon treatment with 4-hydroxy tamoxifen, the cardiomyocytes specifically (due to the cmlc2a promoter driving Cre expression) convert to expressing mCherry. Tamoxifen was injected to adult zebrafish 2 weeks before cardiomyocyte isolation. Cells were cultured for one week until they started to dedifferentiate and divide. Transgene expression was examined in live cells. Due to the beta-actin promoter controlling expression of the mCherry cassette, transgene expression does not get turned off upon dedifferentiation, as is the case in cmlc2a-driven fluorescent tags. (PDF 334 kb)
Supplementary Figure 6
Transfection of primary cardiomyocytes. 0.4 μg pCS+ nlsGFP were transfected with 1 μl Lipofectamine 2000 in Opti-MEM into cardiomyocytes plated 1 day prior to transfection on poly-L-lysine in medium density. The medium was changed after 24 h, and transfection efficiency was examined after 48 h. Scale bars, 100 μm (a) and 50 μm (b). (PDF 272 kb)
Supplementary Table 1
Conditions tested for optimal tissue digestion. (PDF 247 kb)
Supplementary Table 2
Analysis of various parameters for isolation and culture of primary cardiomyocytes. (PDF 286 kb)
Supplementary Table 3
Flow cytometric analysis of yield and purity. (PDF 238 kb)
Supplementary Video 1
Advanced culture of primary cardiomyocytes. 4 weeks after plating, partially dedifferentiated cells can be seen connected to mature rod shaped cardiomyocytes. The whole culture contracts synchronously. (MOV 4522 kb)
Supplementary Video 2
Loss of sarcomeric GFP expression in a 2-weeks culture. Mature cardiomyocytes isolated from cmlc2a:GFP transgenic zebrafish strongly express GFP. Upon dedifferentiation, the sarcomeric apparatus (including cardiac myosin) disassembles, as can be seen by diminishing GFP expression in non-beating cells of flattened shape. (MOV 2722 kb)
Supplementary Video 3
2-days cardiomyocyte culture on fibrin gel. As early as 2 days after plating the cells have formed a network structure and have started beating. (MOV 1658 kb)
Supplementary Video 4
1-week culture of primary cardiomyocytes. The cells have formed network-like connections and started to contract. (MOV 3459 kb)
Supplementary Video 5
Viability decline in long term culture. 5 weeks after plating, the cells contract less frequently and the number of dead cells is increased (see also Supplementary Fig. 2). (MOV 2352 kb)
Supplementary Video 6
10-days culture of primary cardiomyocytes used for ANEP staining. A network of differentiated, spindle-shaped and strongly contracting cells and partially dedifferentiated cells (characterized by their flattened shape and passive beating motion, visible in the centre of the field) has formed. The corresponding pictures showing cell-cell connections by ANEP staining in overview and high magnification are presented in Fig. 3g,g' and Supplementary Fig. 3. An example of a dead cardiomyocyte of elongated appearance with punctuated structures, which is moved only by the underlying live cells, is visible on the right hand side of the culture. (MOV 1447 kb)
Supplementary Video 7
2-weeks culture of primary cardiomyocytes. Flattened, partially dedifferentiated cardiomyocytes are spread out between actively beating mature muscle cells. Mature and dedifferentiated cardiomyocytes remain connected. (MOV 1207 kb)
Supplementary Video 8
Lineage tracing of partially dedifferentiated cells. Expression of mCherry in partially dedifferentiated, flattened cells demonstrates their myocardial origin (see also Supplementary Fig. 5). (MOV 452 kb)
Rights and permissions
About this article
Cite this article
Sander, V., Suñe, G., Jopling, C. et al. Isolation and in vitro culture of primary cardiomyocytes from adult zebrafish hearts. Nat Protoc 8, 800–809 (2013). https://doi.org/10.1038/nprot.2013.041
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2013.041
This article is cited by
-
Nanomaterials based flexible devices for monitoring and treatment of cardiovascular diseases (CVDs)
Nano Research (2023)
-
Characterisation of the single-cell human cardiomyocytes taken from the excess heart tissue of the right ventricular outlet in congenital heart disease
Cell and Tissue Banking (2022)
-
Ultrasound-targeted microbubble destruction (UTMD)-mediated miR-150-5p attenuates oxygen and glucose deprivation-induced cardiomyocyte injury by inhibiting TTC5 expression
Molecular Biology Reports (2022)
-
Fosl1 is vital to heart regeneration upon apex resection in adult Xenopus tropicalis
npj Regenerative Medicine (2021)
-
Artificial cell membrane binding thrombin constructs drive in situ fibrin hydrogel formation
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.