Abstract
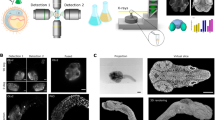
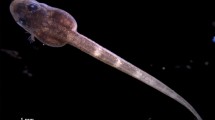
X-ray phase-contrast microtomography (XPCμT) is a label-free, high-resolution imaging modality for analyzing early development of vertebrate embryos in vivo by using time-lapse sequences of 3D volumes. Here we provide a detailed protocol for applying this technique to study gastrulation in Xenopus laevis (African clawed frog) embryos. In contrast to μMRI, XPCμT images optically opaque embryos with subminute temporal and micrometer-range spatial resolution. We describe sample preparation, culture and suspension of embryos, tomographic imaging with a typical duration of 2 h (gastrulation and neurulation stages), intricacies of image pre-processing, phase retrieval, tomographic reconstruction, segmentation and motion analysis. Moreover, we briefly discuss our present understanding of X-ray dose effects (heat load and radiolysis), and we outline how to optimize the experimental configuration with respect to X-ray energy, photon flux density, sample-detector distance, exposure time per tomographic projection, numbers of projections and time-lapse intervals. The protocol requires an interdisciplinary effort of developmental biologists for sample preparation and data interpretation, X-ray physicists for planning and performing the experiment and applied mathematicians/computer scientists/physicists for data processing and analysis. Sample preparation requires 9–48 h, depending on the stage of development to be studied. Data acquisition takes 2–3 h per tomographic time-lapse sequence. Data processing and analysis requires a further 2 weeks, depending on the availability of computing power and the amount of detail required to address a given scientific problem.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
References
Gurdon, J.B. Adult frogs derived from the nuclei of single somatic cells. Dev. Biol. 4, 256–273 (1962).
Gurdon, J.B. Multiple genetically-identical frogs. J. Heredity 53, 4–9 (1962).
Keller, R., Davidson, L.A. & Shook, D.R. How are we shaped: the biomechanics of gastrulation. Differentiation 71, 171–205 (2003).
Moosmann, J. et al. X-ray phase-contrast in vivo microtomography probes novel aspects of Xenopus gastrulation. Nature 497, 374–377 (2013).
Tuft, P.H. The uptake and distribution of water in the embryo of Xenopus laevis (Daudin). J. Exp. Biol. 39, 1–19 (1962).
Brox, T., Bruhn, A., Papenberg, N. & Weickert, J. High-accuracy optical flow estimation based on a theory for warping. Lect. Notes in Comp. Sci. 3024, 25–36 (2004).
Keller, R., Shook, D. & Skoglund, P. The forces that shape embryos: physical aspects of convergent extension by cell intercalation. Phys. Biol. 5, 1–23 (2008).
Paganin, D., Mayo, S.C., Miller, P.R. & Wilkins, S.W. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J. Microsc. 206, 33–40 (2002).
Wu, X., Liu, H. & Yan, A. X-ray phase-attenuation duality and phase retrieval. Opt. Lett. 30, 379–381 (2005).
Ruffins, S.W., Russells, E.J. & Fraser, S.E. Towards a Tralfamadorian view of the embryo: multidimensional imaging of development. Curr. Opin. Neurobiol. 12, 580–586 (2002).
Huisken, J., Swoger, J., DelBene, F., Wittbrodt, J. & Stelzer, E.H.K. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science 305, 1007–1009 (2004).
Keller, Ph.J., Schmidt, A.D., Wittbrodt, J. & Stelzer, E.H.K. Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science 322, 1065–1069 (2008).
Papan, C., Velan, S.S., Fraser, S.E. & Jacobs, R.E. 3D time-lapse analysis of Xenopus gastrulation movements using μMRI. Dev. Biol. 235, 189 (2001).
Kültz, D. Molecular and evolutionary basis of the cellular stress response. Ann. Rev. Physiol. 67, 225–257 (2005).
Howells, M.R. et al. An assessment of the resolution limitation due to radiation-damage in X-ray diffraction microscopy. J. Electr. Spectr. Rel. Phen. 170, 4–12 (2009).
Batenburg, K.J. & Plantagie, L. Fast approximation of algebraic reconstruction methods for tomography. IEEE Trans. Image Process. 21, 3649–3658 (2012).
Snigirev, A., Snigireva, I., Kohn, V., Kuznetsov, S. & Schelokov, I. On the possibilities of X-ray phase contrast microimaging by coherent high-energy synchrotron radiation. Rev. Sci. Instrum. 66, 5486–5493 (1995).
Wilkins, S.W., Gureyev, T.E., Gao, D., Pogany, A. & Stevenson, A.W. Phase-contrast imaging using polychromatic hard X-rays. Nature 384, 335–338 (1996).
Nugent, K.A., Gureyev, T.E., Cookson, D.F., Paganin, D.M. & Barnea, Z. Quantitative phase imaging using hard X rays. Phys. Rev. Lett. 77, 2961–2964 (1996).
Hofmann, R., Moosmann, J. & Baumbach, T. Criticality in single-distance phase retrieval. Opt. Express 19, 25881–25890 (2011).
Moosmann, J., Hofmann, R. & Baumbach, T. Single-distance phase retrieval at large phase shifts. Opt. Express 19, 12066–12073 (2011).
Guigay, J.-P. Fourier transform analysis of Fresnel diffraction patterns and in-line holograms. Optik 49, 121–125 (1977).
Bleuet, P. et al. A hard X-ray nanoprobe for scanning and projection nanotomography. Rev. Sci. Instr. 80, 056101 (2009).
Amaya, E. et al. Techniques and probes for the study of Xenopus tropicalis development. Dev. Dyn. 225, 499–510.
Sive, H.L., Grainger, R.M. & Harland, R.M. Early Development of Xenopus laevis, a Laboratory Manual (Cold Spring Harbor Laboratory Press, 2000).
Nieuwkoop, P.D. & Faber, J. Normal Table of Xenopus laevis (Daudin). North-Holland Publishing Co. (1956).
Henke, B.L., Gullikson, E.M. & Davis, J.C. X-ray interactions: photoabsorption, scattering, transmission, and reflection at E = 50-30000 eV, Z = 1-92. Atom. Data Nucl. Data Tables 54, 181–342 (1993).
Martin, T. et al. LSO-based single crystal film scintillator for synchrotron-based hard X-ray micro-imaging. IEEE Trans. Nucl. Sci. 56, 1412–1418 (2009).
Schneider, C.A., Rasband, W.S. & Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Weickert, J. & Schnörr, C. Variational optic ow computation with a spatio-temporal smoothness constraint. J. Math. Imaging Vision 14, 245–255 (2001).
Sun, D., Roth, S. & Black, M. Secrets of optical flow estimation and their principles. IEEE Conf. Comput. Vision Pattern Recog. 13–18 June 2010 2432–2439 (2010).
Rack, A. et al. Comparative study of multilayers used in monochromators for synchrotron-based coherent hard X-ray imaging. J. Synchr. Rad. 17, 496–451 (2010).
Acknowledgements
We thank T. van de Kamp for his help visualizing the setup and the sample-holder preparation, as well as F. de Carlo for allocating beam time at 2-BM-B station of APS, Argonne National Laboratory. The use of the APS, an Office of Science User Facility operated for the US Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the US DOE under contract no. DE-AC02-06CH11357. J.K.'s Young Investigator Group received financial support from the 'Concept for the Future' program of Karlsruhe Institute of Technology within the framework of the German Excellence Initiative. This research partially was funded by the German Federal Ministry of Education and Research under grant nos. 05K12CK2 and 05K12VH1, as well as by COST action MP1207.
Author information
Authors and Affiliations
Contributions
R.H. drafted the manuscript, and J.M., J.K. and A.E. contributed text. Figures were prepared by J.M., A.E and J.K. V.W., T.B., C.L., M.S.P. and X.X. provided critical review. All authors contributed to the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Moosmann, J., Ershov, A., Weinhardt, V. et al. Time-lapse X-ray phase-contrast microtomography for in vivo imaging and analysis of morphogenesis. Nat Protoc 9, 294–304 (2014). https://doi.org/10.1038/nprot.2014.033
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.033
This article is cited by
-
First record of Jacobsoniidae (Coleoptera) on the African continent in Holocene copal from Tanzania: biogeography since the Cretaceous
Scientific Reports (2023)
-
Simulated biomechanical performance of morphologically disparate ant mandibles under bite loading
Scientific Reports (2023)
-
Comparative study of calcification in human choroid plexus, pineal gland, and habenula
Cell and Tissue Research (2023)
-
The velvet worm brain unveils homologies and evolutionary novelties across panarthropods
BMC Biology (2022)
-
A chondrule formation experiment aboard the ISS: microtomography, scanning electron microscopy and Raman spectroscopy on Mg\(_2\)SiO\(_4\) dust aggregates
Physics and Chemistry of Minerals (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.