Abstract
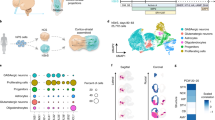
The ability to generate human neurons of specific subtypes of clinical importance offers experimental platforms that may be instrumental for disease modeling. We recently published a study demonstrating the use of neuronal microRNAs (miRNAs) and transcription factors to directly convert human fibroblasts to a highly enriched population of striatal medium spiny neurons (MSNs), a neuronal subpopulation that has a crucial role in motor control and harbors selective susceptibility to cell death in Huntington's disease. Here we describe a stepwise protocol for the generation of MSNs by direct neuronal conversion of human fibroblasts in 30 d. We provide descriptions of cellular behaviors during reprogramming and crucial steps involved in gene delivery, cell adhesion and culturing conditions that promote cell survival. Our protocol offers a unique approach to combine microRNAs and transcription factors to guide the neuronal conversion of human fibroblasts toward a specific neuronal subtype.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
International Basal Ganglia Society. The Basal Ganglia VI (eds. Graybiel, A.M., DeLong, M.R. & Kitai, S.T.) (Kluwer Academic/Plenum Pub., 2003).
Albin, R.L., Young, A.B. & Penney, J.B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375 (1989).
Gerfen, C.R. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu. Rev. Neurosci. 15, 285–320 (1992).
Yoo, A.S. et al. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 476, 228–231 (2011).
Victor, M.B. et al. Generation of human striatal neurons by microRNA-dependent direct conversion of fibroblasts. Neuron 84, 311–323 (2014).
Zhang, N., An, M.C., Montoro, D. & Ellerby, L.M. Characterization of human Huntington's disease cell model from induced pluripotent stem cells. PLoS Curr. 2, RRN1193 (2010).
Ma, L. et al. Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell 10, 455–464 (2012).
Delli Carri, A. et al. Developmentally coordinated extrinsic signals drive human pluripotent stem cell differentiation toward authentic DARPP-32+ medium-sized spiny neurons. Development 140, 301–312 (2013).
HD iPSC Consortium. Induced pluripotent stem cells from patients with Huntington's disease show CAG-repeat-expansion-associated phenotypes. Cell Stem Cell 11, 264–278 (2012).
Arber, C. et al. Activin A directs striatal projection neuron differentiation of human pluripotent stem cells. Development 142, 1375–1386 (2015).
Aubry, L. et al. Striatal progenitors derived from human ES cells mature into DARPP32 neurons in vitro and in quinolinic acid-lesioned rats. Proc. Natl. Acad. Sci. USA 105, 16707–16712 (2008).
Pang, Z.P. et al. Induction of human neuronal cells by defined transcription factors. Nature 476, 220–223 (2011).
Vierbuchen, T. et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 (2010).
Caiazzo, M. et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 476, 224–227 (2011).
Xue, Y. et al. Direct conversion of fibroblasts to neurons by reprogramming PTB-regulated microRNA circuits. Cell 152, 82–96 (2013).
Son, E.Y. et al. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell 9, 205–218 (2011).
Wainger, B.J. et al. Modeling pain in vitro using nociceptor neurons reprogrammed from fibroblasts. Nat. Neurosci. 18, 17–24 (2015).
Nightingale, S.J. et al. Transient gene expression by nonintegrating lentiviral vectors. Mol. Ther. 13, 1121–1132 (2006).
Yanez-Munoz, R.J. et al. Effective gene therapy with nonintegrating lentiviral vectors. Nat. Med. 12, 348–353 (2006).
Chambers, S.M. & Studer, L. Cell fate plug and play: direct reprogramming and induced pluripotency. Cell 145, 827–830 (2011).
Makeyev, E.V., Zhang, J., Carrasco, M.A. & Maniatis, T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol. Cell 27, 435–448 (2007).
Packer, A.N., Xing, Y., Harper, S.Q., Jones, L. & Davidson, B.L. The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington's disease. J. Neurosci. 28, 14341–14346 (2008).
Visvanathan, J., Lee, S., Lee, B., Lee, J.W. & Lee, S.K. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 21, 744–749 (2007).
Yoo, A.S., Staahl, B.T., Chen, L. & Crabtree, G.R. MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature 460, 642–646 (2009).
Conaco, C., Otto, S., Han, J.J. & Mandel, G. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc. Natl. Acad. Sci. USA 103, 2422–2427 (2006).
Kutner, R.H., Zhang, X.Y. & Reiser, J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat. Protoc. 4, 495–505 (2009).
Arlotta, P., Molyneaux, B.J., Jabaudon, D., Yoshida, Y. & Macklis, J.D. Ctip2 controls the differentiation of medium spiny neurons and the establishment of the cellular architecture of the striatum. J. Neurosci. 28, 622–632 (2008).
Herigstad, B., Hamilton, M. & Heersink, J. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 44, 121–129 (2001).
Fedoroff, S. & Richardson, A. Protocols for Neural Cell Culture 3rd edn. (Humana Press, 2001).
Lobo, M.K., Karsten, S.L., Gray, M., Geschwind, D.H. & Yang, X.W. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat. Neurosci. 9, 443–452 (2006).
Ouimet, C.C. & Greengard, P. Distribution of DARPP-32 in the basal ganglia: an electron microscopic study. J. Neurocytol. 19, 39–52 (1990).
Graybiel, A.M., Aosaki, T., Flaherty, A.W. & Kimura, M. The basal ganglia and adaptive motor control. Science 265, 1826–1831 (1994).
Deacon, T.W., Pakzaban, P. & Isacson, O. The lateral ganglionic eminence is the origin of cells committed to striatal phenotypes: neural transplantation and developmental evidence. Brain Res. 668, 211–219 (1994).
Acknowledgements
We thank all members of the Yoo laboratory for helpful suggestions. M.B.V. is supported by a National Science Foundation Graduate Research Fellowship (DGE-1143954). A.S.Y. is supported by a US National Institutes of Health (NIH) Director's Innovator Award (DP2), and awards from the Mallinckrodt, Jr. Foundation and the Ellison Medical Foundation, as well as a Presidential Early Career Award for Scientists and Engineers (PECASE).
Author information
Authors and Affiliations
Contributions
M.R., M.B.V. and A.S.Y. designed and performed experiments, and wrote the manuscript. Y.L. contributed to Table 1. D.A. contributed to Supplementary Information.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Vector maps of all lentiviral plasmids used to generate MSNs.
Plasmids maps and their full sequences can be obtained directly from Addgene at addgene.org/Andrew_Yoo. All plasmids are Ampicillin resistant. Mammalian selection resistance is denominated as follows; N144 – Hygromycin, N174 – Neomycin, N106 – Blasticidin.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 (PDF 294 kb)
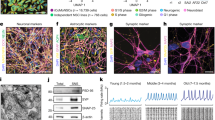
Live imaging of neonatal fibroblasts during microRNA-mediated reprogramming.
Cells were re-plated at PID 3 and imaged every three hours from PID 7 to PID 21. Time stamp of the video is indicative of time since re-plating, and not post-transduction days. The black arrow indicates the cell shown in Figure 5 undergoing reprogramming. Other colored arrows were added to aid the tracking of additional cells during reprogramming. Due to a small degree of movement of the coverslip, the arrows are intended to help viewers follow single cells over time. (MOV 24535 kb)
Rights and permissions
About this article
Cite this article
Richner, M., Victor, M., Liu, Y. et al. MicroRNA-based conversion of human fibroblasts into striatal medium spiny neurons. Nat Protoc 10, 1543–1555 (2015). https://doi.org/10.1038/nprot.2015.102
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2015.102
This article is cited by
-
Longitudinal modeling of human neuronal aging reveals the contribution of the RCAN1–TFEB pathway to Huntington’s disease neurodegeneration
Nature Aging (2023)
-
Age-related Huntington’s disease progression modeled in directly reprogrammed patient-derived striatal neurons highlights impaired autophagy
Nature Neuroscience (2022)
-
Alzheimer’s in a dish – induced pluripotent stem cell-based disease modeling
Translational Neurodegeneration (2019)
-
Modeling Polyglutamine Expansion Diseases with Induced Pluripotent Stem Cells
Neurotherapeutics (2019)
-
Striatal neurons directly converted from Huntington’s disease patient fibroblasts recapitulate age-associated disease phenotypes
Nature Neuroscience (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.