Abstract
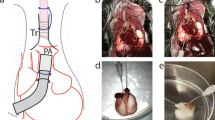
Blood vessels are crucial for the normal development, lifelong repair and homeostasis of tissues. Recently, vascular progenitor cell–driven 'postnatal vasculogenesis' has been suggested as an important mechanism that contributes to new blood vessel formation and organ repair. Among several described progenitor cell types that contribute to blood vessel formation, endothelial colony-forming cells (ECFCs) have received widespread attention as lineage-specific 'true' vascular progenitors. Here we describe a protocol for the isolation of pulmonary microvascular ECFCs from human and rat lung tissue. Our technique takes advantage of an earlier protocol for the isolation of circulating ECFCs from the mononuclear cellular fraction of peripheral blood. We adapted the earlier protocol to isolate resident ECFCs from the distal lung tissue. After enzymatic dispersion of rat or human lung samples into a cellular suspension, CD31-expressing cells are positively selected using magnetic-activated cell sorting and plated in endothelial-specific growth conditions. The colonies arising after 1–2 weeks in culture are carefully separated and expanded to yield pure ECFC cultures after a further 2–3 weeks. The resulting cells demonstrate the defining characteristics of ECFCs such as (i) 'cobblestone' morphology of cultured cell monolayers; (ii) acetylated low-density lipoprotein uptake and Ulex europaeus lectin binding; (iii) tube-like network formation in Matrigel; (iv) expression of endothelial cell–specific surface markers and the absence of hematopoietic or myeloid surface antigens; (v) self-renewal potential displayed by the most proliferative cells; and (vi) contribution to de novo vessel formation in an in vivo mouse implant model. Assuming typical initial cell adhesion and proliferation rates, the entire procedure can be completed within 4 weeks. Isolation and culture of lung vascular ECFCs will allow assessment of the functional state of these cells in experimental and human lung diseases, providing newer insights into their pathophysiological mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
02 December 2015
In the version of this article initially published, the third author's last name was spelled incorrectly. The spelling was initially 'Zong' but should have been 'Zhong'. The error has been corrected in the HTML and PDF versions of the article.
References
Murray, C.J. & Lopez, A.D. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 349, 1498–1504 (1997).
Liebow, A.A. Pulmonary emphysema with special reference to vascular changes. Am. Rev. Respir. Dis. 80, 67–93 (1959).
Sluiter, I. et al. Vascular abnormalities in human newborns with pulmonary hypertension. Expert Rev. Respir. Med. 5, 245–256 (2011).
Thebaud, B. & Abman, S.H. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am. J. Respir. Crit. Care Med. 175, 978–985 (2007).
Voelkel, N.F., Douglas, I.S. & Nicolls, M. Angiogenesis in chronic lung disease. Chest 131, 874–879 (2007).
Asahara, T. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967 (1997).
Asahara, T. Cell therapy and gene therapy using endothelial progenitor cells for vascular regeneration. Handb. Exp. Pharmacol. 2007, 181–194 (2007).
Khoo, C.P., Pozzilli, P. & Alison, M.R. Endothelial progenitor cells and their potential therapeutic applications. Regen. Med. 3, 863–876 (2008).
Kirton, J.P. & Xu, Q. Endothelial precursors in vascular repair. Microvasc. Res. 79, 193–199 (2010).
Steinmetz, M., Nickenig, G. & Werner, N. Endothelial-regenerating cells: an expanding universe. Hypertension 55, 593–599 (2010).
Borghesi, A. et al. Circulating endothelial progenitor cells and diseases of the preterm infant. Minerva Pediatr. 62, 21–23 (2010).
Huertas, A. & Palange, P. Circulating endothelial progenitor cells and chronic pulmonary diseases. Eur. Respir. J. 37, 426–431 (2011).
Ingram, D.A. et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 104, 2752–2760 (2004).
Yoder, M.C. Is endothelium the origin of endothelial progenitor cells? Arterioscler. Thromb. Vasc. Biol. 30, 1094–1103 (2010).
Ingram, D.A. et al. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood 105, 2783–2786 (2005).
Prater, D.N., Case, J., Ingram, D.A. & Yoder, M.C. Working hypothesis to redefine endothelial progenitor cells. Leukemia 21, 1141–1149 (2007).
Alphonse, R.S. et al. Existence, functional impairment, and lung repair potential of endothelial colony-forming cells in oxygen-induced arrested alveolar growth. Circulation 129, 2144–2157 (2014).
Mead, L.E., Prater, D., Yoder, M.C. & Ingram, D.A. Isolation and characterization of endothelial progenitor cells from human blood. Curr. Protoc. Stem Cell Biol. 6, 2C.1.1–2C.1.27 (2008).
King, J. et al. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc. Res. 67, 139–151 (2004).
Alvarez, D.F. et al. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am. J. Physiol. Lung Cell Mol. Physiol. 294, L419–L430 (2008).
Schniedermann, J. et al. Mouse lung contains endothelial progenitors with high capacity to form blood and lymphatic vessels. BMC Cell Biol. 11, 50 (2010).
May, T. et al. Establishment of murine cell lines by constitutive and conditional immortalization. J. Biotechnol. 120, 99–110 (2005).
Rafii, S. et al. Isolation and characterization of human bone marrow microvascular endothelial cells: hematopoietic progenitor cell adhesion. Blood 84, 10–19 (1994).
de Fougerolles, A.R., Stacker, S.A., Schwarting, R. & Springer, T.A. Characterization of ICAM-2 and evidence for a third counter-receptor for LFA-1. J. Exp. Med. 174, 253–267 (1991).
Fang, S., Wei, J., Pentinmikko, N., Leinonen, H. & Salven, P. Generation of functional blood vessels from a single c-kit+ adult vascular endothelial stem cell. PLoS Biol. 10, e1001407 (2012).
Wu, S., Zhou, C., King, J.A. & Stevens, T. A unique pulmonary microvascular endothelial cell niche revealed by Weibel-Palade bodies and Griffonia simplicifolia. Pulm. Circ. 4, 110–115 (2014).
Acknowledgements
This work was supported by the Canadian Institutes of Health Research (CIHR) and the Canadian Heart and Stroke Foundation. R.S.A. was supported by Alberta Innovates-Health Solutions (AIHS). A.V. was supported by a studentship from the Mazankowski Heart Institute. B.T. was supported by the Canada Foundation for Innovation and the Stollery Children's Hospital Foundation. The work was also supported in part by funds from the Riley Children's Foundation (to M.C.Y.).
Author information
Authors and Affiliations
Contributions
R.S.A. developed the protocol design, troubleshot and perfected the steps of the procedure, performed experiments, analyzed data and wrote the manuscript. A.V. and S.Z. performed the experiments, helped with experimental design and contributed to manuscript preparation. S.M. and R.O. helped with human tissue acquisition and experimental design. M.C.Y. contributed to protocol design, guided troubleshooting and edited the manuscript. B.T. supervised the project, contributed to experimental design, guided protocol development and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1 and 2 (PDF 144 kb)
Rights and permissions
About this article
Cite this article
Alphonse, R., Vadivel, A., Zhong, S. et al. The isolation and culture of endothelial colony-forming cells from human and rat lungs. Nat Protoc 10, 1697–1708 (2015). https://doi.org/10.1038/nprot.2015.107
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2015.107
This article is cited by
-
Mitigating the non-specific uptake of immunomagnetic microparticles enables the extraction of endothelium from human fat
Communications Biology (2021)
-
Clonally selected primitive endothelial cells promote occlusive pulmonary arteriopathy and severe pulmonary hypertension in rats exposed to chronic hypoxia
Scientific Reports (2020)
-
Hepatocyte-derived exosomal MiR-194 activates PMVECs and promotes angiogenesis in hepatopulmonary syndrome
Cell Death & Disease (2019)
-
Tissue regeneration using endothelial colony-forming cells: promising cells for vascular repair
Pediatric Research (2018)
-
Stem cell biology and regenerative medicine for neonatal lung diseases
Pediatric Research (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.