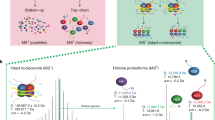
Abstract
Rapid immunoprecipitation mass spectrometry of endogenous protein (RIME) is a method that allows the study of protein complexes, in particular chromatin and transcription factor complexes, in a rapid and robust manner by mass spectrometry (MS). The method can be used in parallel with chromatin immunoprecipitation–sequencing (ChIP-seq) experiments to provide information on both the cistrome and interactome for a given protein. The method uses formaldehyde fixation to stabilize protein complexes. By using antibodies against the endogenous target, the cross-linked complex is immunoprecipitated, rigorously washed, and then digested into peptides while avoiding antibody contamination (on-bead digestion). By using this method, MS identification of the target protein and several dozen interacting proteins is possible using a 100-min LC-MS/MS run. The protocol does not require substantial proteomics expertise, and it typically takes 2–3 d from the collection of material to results.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Alberts, B. The cell as a collection of protein machines: preparing the next generation of molecular biologists. Cell 92, 291–294 (1998).
Dunham, W.H., Mullin, M. & Gingras, A.C. Affinity-purification coupled to mass spectrometry: basic principles and strategies. Proteomics 12, 1576–1590 (2012).
Giambruno, R. et al. Affinity purification strategies for proteomic analysis of transcription factor complexes. J. Proteome Res. 12, 4018–4027 (2013).
Greenberg, R.A. et al. Multifactorial contributions to an acute DNA damage response by BRCA1/BARD1-containing complexes. Genes Dev. 20, 34–46 (2006).
Kocher, T. & Superti-Furga, G. Mass spectrometry-based functional proteomics: from molecular machines to protein networks. Nat. Methods 4, 807–815 (2007).
Marcon, E. et al. Assessment of a method to characterize antibody selectivity and specificity for use in immunoprecipitation. Nat. Methods 12, 725–731 (2015).
Morris, J.H. et al. Affinity purification–mass spectrometry and network analysis to understand protein-protein interactions. Nat. Protoc. 9, 2539–2554 (2014).
Gavin, A.C. et al. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415, 141–147 (2002).
Ho, Y. et al. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 415, 180–183 (2002).
Krogan, N.J. et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 440, 637–643 (2006).
Babu, M., Krogan, N.J., Awrey, D.E., Emili, A. & Greenblatt, J.F. Systematic characterization of the protein interaction network and protein complexes in Saccharomyces cerevisiae using tandem affinity purification and mass spectrometry. Methods Mol. Biol. 548, 187–207 (2009).
Guruharsha, K.G. et al. A protein complex network of Drosophila melanogaster. Cell 147, 690–703 (2011).
Rhee, D.Y. et al. Transcription factor networks in Drosophila melanogaster. Cell Rep. 8, 2031–2043 (2014).
Rubio, V. et al. An alternative tandem affinity purification strategy applied to Arabidopsis protein complex isolation. Plant J. 41, 767–778 (2005).
Van Leene, J. et al. A tandem affinity purification-based technology platform to study the cell cycle interactome in Arabidopsis thaliana. Mol. Cell. Proteomics 6, 1226–1238 (2007).
Van Leene, J. et al. An improved toolbox to unravel the plant cellular machinery by tandem affinity purification of Arabidopsis protein complexes. Nat. Protoc. 10, 169–187 (2015).
Ogawa, H., Ishiguro, K., Gaubatz, S., Livingston, D.M. & Nakatani, Y. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science 296, 1132–1136 (2002).
Ewing, R.M. et al. Large-scale mapping of human protein-protein interactions by mass spectrometry. Mol. Syst. Biol. 3, 89 (2007).
Sowa, M.E., Bennett, E.J., Gygi, S.P. & Harper, J.W. Defining the human deubiquitinating enzyme interaction landscape. Cell 138, 389–403 (2009).
Zhou, Z., Licklider, L.J., Gygi, S.P. & Reed, R. Comprehensive proteomic analysis of the human spliceosome. Nature 419, 182–185 (2002).
Huttlin, E.L. et al. The BioPlex network: a systematic exploration of the human interactome. Cell 162, 425–440 (2015).
Tsai, A. & Carstens, R.P. An optimized protocol for protein purification in cultured mammalian cells using a tandem affinity purification approach. Nat. Protoc. 1, 2820–2827 (2006).
Malovannaya, A. et al. Analysis of the human endogenous coregulator complexome. Cell 145, 787–799 (2011).
Malovannaya, A. et al. Streamlined analysis schema for high-throughput identification of endogenous protein complexes. Proc. Natl. Acad. Sci. USA 107, 2431–2436 (2010).
Brohee, S., Faust, K., Lima-Mendez, G., Vanderstocken, G. & van Helden, J. Network analysis tools: from biological networks to clusters and pathways. Nat. Protoc. 3, 1616–1629 (2008).
Bensimon, A., Heck, A.J. & Aebersold, R. Mass spectrometry-based proteomics and network biology. Annu. Rev. Biochem. 81, 379–405 (2012).
Drewes, G. & Bouwmeester, T. Global approaches to protein-protein interactions. Curr. Opin. Cell Biol. 15, 199–205 (2003).
Kutzera, J. et al. Inferring protein-protein interaction complexes from immunoprecipitation data. BMC Res. Notes 6, 468 (2013).
Chiang, T. & Scholtens, D. A general pipeline for quality and statistical assessment of protein interaction data using R and Bioconductor. Nat. Protoc. 4, 535–546 (2009).
Selbach, M. & Mann, M. Protein interaction screening by quantitative immunoprecipitation combined with knockdown (QUICK). Nat. Methods 3, 981–983 (2006).
Trinkle-Mulcahy, L. Resolving protein interactions and complexes by affinity purification followed by label-based quantitative mass spectrometry. Proteomics 12, 1623–1638 (2012).
Trinkle-Mulcahy, L. et al. Identifying specific protein interaction partners using quantitative mass spectrometry and bead proteomes. J. Cell Biol. 183, 223–239 (2008).
Tackett, A.J. et al. I-DIRT, a general method for distinguishing between specific and nonspecific protein interactions. J. Proteome Res. 4, 1752–1756 (2005).
Mellacheruvu, D. et al. The CRAPome: a contaminant repository for affinity purification-mass spectrometry data. Nat. Methods 10, 730–736 (2013).
Mohammed, H. et al. Endogenous purification reveals GREB1 as a key estrogen receptor regulatory factor. Cell Rep. 3, 342–349 (2013).
Mohammed, H. et al. Progesterone receptor modulates ER action in breast cancer. Nature 523, 313–317 (2015).
Srinivasa, S., Ding, X. & Kast, J. Formaldehyde cross-linking and structural proteomics: bridging the gap. Methods 89, 91–98 (2015).
Sutherland, B.W., Toews, J. & Kast, J. Utility of formaldehyde cross-linking and mass spectrometry in the study of protein-protein interactions. J. Mass Spectrom. 43, 699–715 (2008).
Vasilescu, J., Guo, X. & Kast, J. Identification of protein-protein interactions using in vivo cross-linking and mass spectrometry. Proteomics 4, 3845–3854 (2004).
Ramos-Vara, J.A. Principles and methods of immunohistochemistry. Methods Mol. Biol. 691, 83–96 (2011).
Schmidt, D. et al. ChIP-seq: using high-throughput sequencing to discover protein-DNA interactions. Methods 48, 240–248 (2009).
Latos, P.A. et al. Fgf and Esrrb integrate epigenetic and transcriptional networks that regulate self-renewal of trophoblast stem cells. Nat. Commun. 6, 7776 (2015).
Xu, Y. et al. LMTK3 represses tumor suppressor-like genes through chromatin remodeling in breast cancer. Cell Rep. 12, 837–849 (2015).
Sanders, D.A. et al. FOXM1 binds directly to non-consensus sequences in the human genome. Genome Biol. 16, 130 (2015).
Ji, Z. et al. The forkhead transcription factor FOXK2 acts as a chromatin targeting factor for the BAP1-containing histone deubiquitinase complex. Nucleic Acids Res. 42, 6232–6242 (2014).
Ali, S. & Coombes, R.C. Endocrine-responsive breast cancer and strategies for combating resistance. Nat. Rev. Cancer 2, 101–112 (2002).
Metivier, R. et al. Estrogen receptor- directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115, 751–763 (2003).
Okada, M. et al. Switching of chromatin-remodelling complexes for oestrogen receptor-α. EMBO Rep. 9, 563–568 (2008).
Metz, B. et al. Identification of formaldehyde-induced modifications in proteins: reactions with insulin. Bioconjug. Chem. 17, 815–822 (2006).
Metz, B. et al. Identification of formaldehyde-induced modifications in proteins: reactions with model peptides. J. Biol. Chem. 279, 6235–6243 (2004).
Byrum, S.D., Raman, A., Taverna, S.D. & Tackett, A.J. ChAP-MS: a method for identification of proteins and histone posttranslational modifications at a single genomic locus. Cell Rep. 2, 198–205 (2012).
Pourfarzad, F. et al. Locus-specific proteomics by TChP: targeted chromatin purification. Cell Rep. 4, 589–600 (2013).
Soldi, M. & Bonaldi, T. The ChroP approach combines ChIP and mass spectrometry to dissect locus-specific proteomic landscapes of chromatin. J. Vis. Exp. (2014).
Wang, C.I. et al. Chromatin proteins captured by ChIP–mass spectrometry are linked to dosage compensation in Drosophila. Nat. Struct. Mol. Biol. 20, 202–209 (2013).
Engelen, E. et al. Proteins that bind regulatory regions identified by histone modification chromatin immunoprecipitations and mass spectrometry. Nat. Commun. 6, 7155 (2015).
Ji, X. et al. Chromatin proteomic profiling reveals novel proteins associated with histone-marked genomic regions. Proc. Natl. Acad. Sci. USA 112, 3841–3846 (2015).
Boyer, L.A. et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122, 947–956 (2005).
Acknowledgements
We acknowledge the support of the University of Cambridge and Cancer Research UK. J.S.C. is supported by a European Research Council (ERC) starting grant and a European Molecular Biology Organization (EMBO) Young investigator award.
Author information
Authors and Affiliations
Contributions
H.M., J.S.C. and C.S.D. designed the research. H.M., C.T., E.P., G.D.B., J.S.C. and C.S.D. performed the experiments and analyzed the data. H.M. and C.S.D. wrote the manuscript. H.M., C.T., E.P., G.D.B., J.S.C. and C.S.D. edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Mohammed, H., Taylor, C., Brown, G. et al. Rapid immunoprecipitation mass spectrometry of endogenous proteins (RIME) for analysis of chromatin complexes. Nat Protoc 11, 316–326 (2016). https://doi.org/10.1038/nprot.2016.020
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.020
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.