Abstract
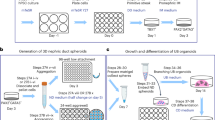
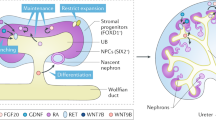
A variety of protocols have been developed that demonstrate the capability to differentiate human pluripotent stem cells (hPSCs) into kidney structures. Our goal was to develop a high-efficiency protocol to generate nephron progenitor cells (NPCs) and kidney organoids to facilitate applications for tissue engineering, disease modeling and chemical screening. Here, we describe a detailed protocol resulting in high-efficiency production (80–90%) of NPCs from hPSCs within 9 d of differentiation. Kidney organoids were generated from NPCs within 12 d with high reproducibility using 96-well plates suitable for chemical screening. The protocol requires skills for culturing hPSCs and careful attention to morphological changes indicative of differentiation. This kidney organoid system provides a platform for studies of human kidney development, modeling of kidney diseases, nephrotoxicity and kidney regeneration. The system provides a model for in vitro study of kidney intracellular and intercompartmental interactions using differentiated human cells in an appropriate nephron and stromal context.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Takasato, M. et al. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat. Cell Biol. 16, 118–126 (2014).
Lam, A.Q. et al. Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. J. Am. Soc. Nephrol. 25, 1211–1225 (2014).
Taguchi, A. et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14, 53–67 (2014).
Vigneau, C. et al. Mouse embryonic stem cell-derived embryoid bodies generate progenitors that integrate long term into renal proximal tubules in vivo. J. Am. Soc. Nephrol. 18, 1709–1720 (2007).
Bruce, S.J. et al. In vitro differentiation of murine embryonic stem cells toward a renal lineage. Differentiation 75, 337–349 (2007).
Morizane, R., Monkawa, T. & Itoh, H. Differentiation of murine embryonic stem and induced pluripotent stem cells to renal lineage in vitro. Biochem. Biophys. Res. Commun. 390, 1334–1339 (2009).
Narayanan, K. et al. Human embryonic stem cells differentiate into functional renal proximal tubular-like cells. Kidney Int. 83, 593–603 (2013).
Morizane, R. et al. Kidney specific protein-positive cells derived from embryonic stem cells reproduce tubular structures in vitro and differentiate into renal tubular cells. PLoS One 8, e64843 (2013).
Mae, S. et al. Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat. Commun. 4, 1367 (2013).
Xia, Y. et al. The generation of kidney organoids by differentiation of human pluripotent cells to ureteric bud progenitor-like cells. Nat. Protoc. 9, 2693–2704 (2014).
Takasato, M. et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564–568 (2015).
Freedman, B.S. et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat. Commun. 6, 8715 (2015).
Morizane, R. et al. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol. 33, 1193–1200 (2015).
Iimura, T. & Pourquie, O. Collinear activation of Hoxb genes during gastrulation is linked to mesoderm cell ingression. Nature 442, 568–571 (2006).
Deschamps, J. & van Nes, J. Developmental regulation of the Hox genes during axial morphogenesis in the mouse. Development 132, 2931–2942 (2005).
Lengerke, C. et al. BMP and Wnt specify hematopoietic fate by activation of the Cdx-Hox pathway. Cell Stem Cell 2, 72–82 (2008).
Hobbs, R.F., Song, H., Huso, D.L., Sundel, M.H. & Sgouros, G. A nephron-based model of the kidneys for macro-to-micro α-particle dosimetry. Phys. Med. Biol. 57, 4403–4424 (2012).
Devuyst, O. et al. Rare inherited kidney diseases: challenges, opportunities, and perspectives. Lancet 383, 1844–1859 (2014).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Sterneckert, J.L., Reinhardt, P. & Scholer, H.R. Investigating human disease using stem cell models. Nat. Rev. Genet. 15, 625–639 (2014).
Taguchi, A. & Nishinakamura, R. Nephron reconstitution from pluripotent stem cells. Kidney Int. 87, 894–900 (2015).
Osafune, K. et al. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat. Biotechnol. 26, 313–315 (2008).
Deen, P.M. et al. Assignment of the human gene for the water channel of renal collecting duct Aquaporin 2 (AQP2) to chromosome 12 region q12-->q13. Cytogenet. Cell Genet. 66, 260–262 (1994).
Humphreys, B.D. et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 176, 85–97 (2010).
Samulowitz, U. et al. Human endomucin: distribution pattern, expression on high endothelial venules, and decoration with the MECA-79 epitope. Am. J. Pathol. 160, 1669–1681 (2002).
Nakagawa, M. et al. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci. Rep. 4, 3594 (2014).
Watanabe, K. et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 25, 681–686 (2007).
Bennett, C.N. et al. Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 277, 30998–31004 (2002).
Vaidya, V.S., Ramirez, V., Ichimura, T., Bobadilla, N.A. & Bonventre, J.V. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am. J. Physiol. Renal Physiol. 290, F517–529 (2006).
Vaidya, V.S. et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat. Biotechnol. 28, 478–485 (2010).
Freedman, B.S. et al. Reduced ciliary polycystin-2 in induced pluripotent stem cells from polycystic kidney disease patients with PKD1 mutations. J. Am. Soc. Nephrol. 24, 1571–1586 (2013).
Acknowledgements
The authors thank N. Gupta for providing the immunohistochemistry images of kidney organoids in Supplementary Figure 1. This study was supported by National Institutes of Health grants R37 DK039773 and R01 DK072381 (to J.V.B.), a Grant-in-Aid for a Japan Society for the Promotion of Science (JSPS) Postdoctoral Fellowship for Research Abroad (to R.M.), a ReproCell Stem Cell Research grant (to R.M.), a Brigham and Women's Hospital Research Excellence Award (to R.M.), a Brigham and Women's Hospital Faculty Career Development Award (to R.M.) and a Harvard Stem Cell Institute Seed grant (to R.M.).
Author information
Authors and Affiliations
Contributions
R.M. and J.V.B. formulated the strategy for this study. R.M. designed and performed experiments. R.M. and J.V.B. wrote the manuscript. J.V.B. helped to design the experiments and to interpret the results.
Corresponding author
Ethics declarations
Competing interests
J.V.B. is a co-inventor on KIM-1 patents that have been licensed by Partners Healthcare to several companies. He has received royalty income from Partners Healthcare. J.V.B. and R.M. are co-inventors on patents (PCT/US16/52350) on organoid technologies that are assigned to Partners Healthcare. J.V.B. or his family has received income for consulting from companies interested in biomarkers: Sekisui, Millennium, Johnson & Johnson and Novartis. J.V.B. is a consultant to Goldfinch Bio. J.V.B. owns equity in Goldfinch Bio.
Integrated supplementary information
Supplementary Figure 1 Immunostaining for interstitial cells and connecting tubules and collecting ducts.
(a) Immunostaining for PDGFRb (platelet derived growth factor receptor beta) and endomucin in 3D kidney organoids. PDGFRβ was assessed by immunohistochemistry in 3D kidney organoids on day 65. Endomucin was evaluated by whole mount staining in 3D kidney organoids on day 24. Arrows indicate endomucin+ endothelia in a glomerular structure. (b) Immunohistochemistry for α-SMA (smooth muscle alpha-actin) in 3D kidney organoids on day 65. There was a very small population of α-SMA+ interstitial cells in kidney organoids. (c) Immunohistochemistry for aquaporin-2 (AQP2) in CDH1+ tubule in 3D kidney organoids on day 35. AQP2+ tubules were found in only CDH1+ tubules, indicating presence of connecting tubules/collecting ducts in kidney organoids. Scale bars: 50 μm.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 (PDF 225 kb)
Rights and permissions
About this article
Cite this article
Morizane, R., Bonventre, J. Generation of nephron progenitor cells and kidney organoids from human pluripotent stem cells. Nat Protoc 12, 195–207 (2017). https://doi.org/10.1038/nprot.2016.170
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.170
This article is cited by
-
HIF-1α promotes kidney organoid vascularization and applications in disease modeling
Stem Cell Research & Therapy (2023)
-
RAAS-deficient organoids indicate delayed angiogenesis as a possible cause for autosomal recessive renal tubular dysgenesis
Nature Communications (2023)
-
HNF1A binds and regulates the expression of SLC51B to facilitate the uptake of estrone sulfate in human renal proximal tubule epithelial cells
Cell Death & Disease (2023)
-
A live-cell image-based machine learning strategy for reducing variability in PSC differentiation systems
Cell Discovery (2023)
-
Directed differentiation of ureteric bud and collecting duct organoids from human pluripotent stem cells
Nature Protocols (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.