Key Points
-
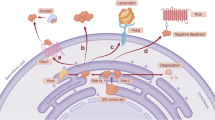
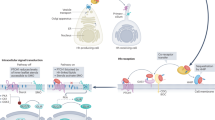
Hedgehog (HH) signalling is required for cell differentiation and organ formation during embryogenesis. In the adult, HH signalling remains active in some organs where it has been implicated in the regulation of stem-cell maintenance and proliferation.
-
HH signalling targets include genes that are important for cell proliferation — proto-oncogenes — as well as growth factors.
-
Misregulation of HH signalling has been shown to cause formation of basal-cell carcinoma and medulloblastoma, and mutations of HH pathway components have been found both in familial and sporadic cases. More recently, small-cell lung cancer (SCLC) and pancreatic adenocarcinoma have been linked to HH signalling, providing a molecular mechanism for these aggressive diseases.
-
Importantly, HH signalling seems to be required not only for cancer initiation but also for tumour growth and survival of medulloblastomas, SCLC and pancreatic adenocarcinoma.
-
HH inhibitors could provide novel therapeutic approaches for treatment of otherwise hard to cure cancer types. Synthetic compounds have been identified that act as HH inhibitors in a very specific manner.
Abstract
The Hedgehog signalling pathway is essential for numerous processes during embryonic development. Members of this family of secreted proteins control cell proliferation, differentiation and tissue patterning in a dose-dependent manner. Although the overall activity of the pathway is diminished after embryogenesis, recent reports show that the pathway remains active in some adult tissues, including adult stem cells in the brain and skin. There is also evidence that uncontrolled activation of the pathway results in specific types of cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Nüsslein-Volhard, C. & Wieschaus, E. Mutations affecting segment number and polarity in Drosophila. Nature 287, 795–801 (1980).
Ingham, P. W. & McMahon, A. P. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15, 3059–3087 (2001).
Marigo, V., Davey, R. A., Zuo, Y., Cunningham, J. M. & Tabin, C. J. Biochemical evidence that patched is the Hedgehog receptor. Nature 384, 176–179 (1996).
Stone, D. M. et al. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature 384, 129–134 (1996).
Goodrich, L. V., Johnson, R. L., Milenkovic, L., McMahon, J. A. & Scott, M. P. Conservation of the hedghog/patched signaling pathway from flies to mice: induction of a mouse patched gene by hedgehog. Genes Dev. 10, 301–312 (1996).
Carpenter, D. et al. Characterization of two patched receptors for the vertebrate hedgehog protein family. Proc. Natl Acad. Sci. USA 95, 13630–13634 (1998).
Motoyama, J. et al. Overlapping and non-overlapping Ptch2 expression with Shh during mouse embryogenesis. Mech. Dev. 78, 81–84 (1998).
Chuang, P. T. & McMahon, A. P. Vertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding protein. Nature 397, 617–621 (1999).
Kalderon, D. Transducing the hedgehog signal. Cell 103, 371–374 (2000).
Ruiz i Altaba, A., Sanchez, P. & Dahmane, N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nature Rev. Cancer 2, 361–372 (2002).
Murone, M. et al. Gli regulation by the opposing activities of fused and suppressor of fused. Nature Cell Biol. 2, 310–312 (2000).
Ingham, P. W. Transducing Hedgehog: the story so far. EMBO J. 17, 3505–3511 (1998).
Chiang, C. et al. Cyclopia and defective axial patterning in mice lacking sonic hedgehog function. Nature 383, 407–413 (1996).
Ericson, J., Briscoe, J., Rashbass, P., van Heyningen, V. & Jessell, T. M. Graded sonic hedgehog signaling and the specification of cell fate in the ventral neural tube. Cold Spring Harb. Symp. Quant. Biol. 62, 451–466 (1997).
Marti, E., Bumcrot, D., Takada, R. & McMahon, A. P. Requirement of 19K form of sonic hedgehog for induction of distinct ventral cell types in CNS explants. Nature 375, 322–325 (1995).
Roelink, H. et al. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell 81, 445–455 (1995).
Hebrok, M. Hedgehog signaling in pancreas development. Mech. Dev. 20, 45–57 (2003).
Apelqvist, A., Ahlgren, U. & Edlund, H. Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr. Biol. 7, 801–804 (1997).
Hebrok, M., Kim, S. K. & Melton, D. A. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev. 12, 1705–1713 (1998).
Hebrok, M., Kim, S. K., St. Jacques, B., McMahon, A. P. & Melton, D. A. Regulation of pancreas development by Hedgehog signaling. Development 127, 4905–4913 (2000).
Thomas, M. K., Rastalsky, N., Lee, J. H. & Habener, J. F. Hedgehog signaling regulation of insulin production by pancreatic β-cells. Diabetes 49, 2039–2047 (2000).
Thomas, M. K., Lee, J. H., Rastalsky, N. & Habener, J. F. Hedgehog signaling regulation of homeodomain protein islet duodenum homeobox-1 expression in pancreatic β-cells. Endocrinology 142, 1033–1040 (2001).
Kawahira, H. et al. Combined activities of Hedgehog signaling inhibitors regulate pancreas development. Development 130, 4871–4879 (2003).
Duman-Scheel, M., Weng, L., Xin, S. & Du, W. Hedgehog regulates cell growth and proliferation by inducing Cyclin D and Cyclin E. Nature 417, 299–304 (2002). Studies in Drosophila show that Hh signalling is involved in regulating cellular proliferation and growth by promoting the transcription of two G1–S cyclins — cyclin D and cyclin E.
Mill, P. et al. Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes Dev. 17, 282–294 (2003).
Barnes, E. A., Kong, M., Ollendorff, V. & Donoghue, D. J. Patched1 interacts with cyclin B1 to regulate cell cycle progression. EMBO J. 20, 2214–2223 (2001).
Fan, H. & Khavari, P. A. Sonic hedgehog opposes epithelial cell cycle arrest. J. Cell Biol. 147, 71–76 (1999).
Oro, A. E. et al. Basal cell carcinomas in mice overexpressing sonic hedgehog. Science 276, 817–821 (1997). Analysis of a mouse model shows that Shh overexpression is sufficient to induce formation of BCC.
Fan, H., Oro, A. E., Scott, M. P. & Khavari, P. A. Induction of basal cell carcinoma features in transgenic human skin expressing Sonic Hedgehog. Nature Med. 3, 788–792 (1997).
Kinzler, K. W. et al. Identification of an amplified, highly expressed gene in a human glioma. Science 236, 70–73 (1987).
Holland, E. C. Gliomagenesis: genetic alterations and mouse models. Nature Rev. Genet. 2, 120–129 (2001).
Dahmane, N., Lee, J., Robins, P., Heller, P. & Ruiz i Altaba, A. Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature 389, 876–881 (1997).
Nilsson, M. et al. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc. Natl Acad. Sci. USA 97, 3438–3443 (2000).
Grachtchouk, M. et al. Basal cell carcinomas in mice overexpressing Gli2 in skin. Nature Genet. 24, 216–217 (2000).
Taylor, M. D. et al. Mutations in SUFU predispose to medulloblastoma. Nature Genet. 31, 306–310 (2002).
Johnson, R. L. et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science 272, 1668–1671 (1996).
Hahn, H. et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell 85, 841–851 (1996).
Goodrich, L. V., Milenkovic, L., Higgins, K. M. & Scott, M. P. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 277, 1109–1113 (1997).
Wolter, M., Reifenberger, J., Sommer, C., Ruzicka, T. & Reifenberger, G. Mutations in the human homologue of the Drosophila segment polarity gene patched (PTCH) in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res. 57, 2581–2585 (1997).
Xie, J. et al. Mutations of the PATCHED gene in several types of sporadic extracutaneous tumors. Cancer Res. 57, 2369–2372 (1997).
Xie, J. et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature 391, 90–92 (1998).
Reifenberger, J. et al. Missense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res. 58, 1798–1803 (1998).
Watkins, D. N. et al. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature 422, 313–317 (2003). Evidence that HH signalling is activated in SCLC and that constitutive activation of the pathway is required for cancer maintenance.
Thayer, S. P. et al. Hedgehog an early and late mediator of pancreatic cancer tumorigenesis. Nature 425, 851–855 (2003). HH pathway misregulation induces formation of pancreatic adenocarcinoma and maintains tumour growth and survival.
Zochbauer-Muller, S., Gazdar, A. F. & Minna, J. D. Molecular pathogenesis of lung cancer. Annu. Rev. Physiol. 64, 681–708 (2002).
Bardeesy, N. & DePinho, R. A. Pancreatic cancer biology and genetics. Nature Rev. Cancer 2, 897–909 (2002).
Chen, J. K., Taipale, J., Cooper, M. K. & Beachy, P. A. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 16, 2743–2748 (2002).
Klimstra, D. S. & Longnecker, D. S. K-ras mutations in pancreatic ductal proliferative lesions. Am. J. Pathol. 145, 1547–1550 (1994).
Day, J. D. et al. Immunohistochemical evaluation of HER-2/neu expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasms. Hum. Pathol. 27, 119–124 (1996).
Dahmane, N. et al. The Sonic Hedgehog–Gli pathway regulates dorsal brain growth and tumorigenesis. Development 128, 5201–5212 (2001). This study shows for the first time that human brain tumour cell lines and primary tumours respond to cyclopamine and that this drug inhibits their proliferation.
Berman, D. M. et al. Widespread requirement for hedgehog ligand stimulation in growth of digestive tract tumours. Nature 425, 846–850 (2003). Evidence that deregulation of HH signalling is a common parameter in several gastrointestinal tumours.
Eberle, M. A. et al. A new susceptibility locus for autosomal dominant pancreatic cancer maps to chromosome 4q32-34. Am. J. Hum. Genet. 70, 1044–1048 (2002).
Chuang, P. T., Kawcak, T. & McMahon, A. P. Feedback control of mammalian Hedgehog signaling by the Hedgehog-binding protein, Hip1, modulates Fgf signaling during branching morphogenesis of the lung. Genes Dev. 17, 342–347 (2003).
Berman, D. M. et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science 297, 1559–1561 (2002). Evidence that Hh signalling is required for the maintenance of medulloblastoma both in cell culture and in allograft experiments in nude mice.
Kenney, A. M., Cole, M. D. & Rowitch, D. H. Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development 130, 15–28 (2003).
Amin, A., Li, Y. & Finkelstein, R. Hedgehog activates the EGF receptor pathway during Drosophila head development. Development 126, 2623–2630 (1999).
Korc, M. et al. Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with concomitant increases in the levels of epidermal growth factor and transforming growth factor α. J. Clin. Invest. 90, 1352–1360 (1992).
Wagner, M. et al. A murine tumour progression model for pancreatic cancer recapitulating the genetic alterations of the human disease. Genes Dev. 15, 286–293 (2001).
McCormick, F. Activators and effectors of ras p21 proteins. Curr. Opin. Genet. Dev. 4, 71–76 (1994).
Treisman, R. Journey to the surface of the cell: Fos regulation and the SRE. EMBO J. 14, 4905–4913 (1995).
Xie, J. et al. A role of PDGFRα in basal cell carcinoma proliferation. Proc. Natl Acad. Sci. USA 98, 9255–9259 (2001).
Nicolas, M. et al. Notch1 functions as a tumour suppressor in mouse skin. Nat Genet 33, 416–421 (2003).
MiYamoto, Y. et al. Notch mediates TGF α-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell 3, 565–576 (2003).
Mullor, J. L., Dahmane, N., Sun, T. & Ruiz i Altaba, A. Wnt signals are targets and mediators of Gli function. Curr. Biol. 11, 769–773 (2001).
Oro, A. E. & Higgins, K. Hair cycle regulation of Hedgehog signal reception. Dev. Biol. 255, 238–248 (2003).
Bonner-Weir, S. & Sharma, A. Pancreatic stem cells. J. Pathol. 197, 519–526 (2002).
Hruban, R. H. et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am. J. Surg. Pathol. 25, 579–586 (2001).
Meszoely, I. M., Means, A. L., Scoggins, C. R. & Leach, S. D. Developmental aspects of early pancreatic cancer. Cancer J. 7, 242–250 (2001).
Ramaswamy, S., Ross, K. N., Lander, E. S. & Golub, T. R. A molecular signature of metastasis in primary solid tumors. Nature Genet. 33, 49–54 (2003).
Keeler, R. F. & Binns, W. Teratogenic compounds of Veratrum californicum (Durand). V. Comparison of cyclopian effects of steroidal alkaloids from the plant and structurally related compounds from other sources. Teratology 1, 5–10 (1968).
Cooper, M. K., Porter, J. A., Young, K. E. & Beachy, P. A. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science 280, 1603–1607 (1998).
Incardona, J. P., Gaffield, W., Kapur, R. P. & Roelink, H. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development 125, 3553–3562. (1998).
Taipale, J. et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature 406, 1005–1009 (2000).
Chen, J. K., Taipale, J., Young, K. E., Maiti, T. & Beachy, P. A. Small molecule modulation of Smoothened activity. Proc. Natl Acad. Sci. USA 99, 14071–14076 (2002).
Williams, J. A. et al. Identification of a small molecule inhibitor of the hedgehog signaling pathway: effects on basal cell carcinoma-like lesions. Proc. Natl Acad. Sci. USA 100, 4616–4621 (2003).
Ericson, J., Morton, S., Kawakami, A., Roelink, H. & Jessell, T. M. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell 87, 661–673 (1996).
Dahmane, N. & Ruiz i Altaba, A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development 126, 3089–3100 (1999).
Wallace, V. A. Purkinjie-cell-derived Sonic Hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 9, 445–448 (1999).
Acknowledgements
We would like to dedicate this manuscript to the memory of Ira Herskowitz, who inspired us to contemplate about the connection between embryonic signalling pathways and cancer. We would like to thank all members of the Hebrok laboratory for stimulating discussions. In particular, we would like to thank P. Heiser and J. Lau as well as H. Kawahira, D. Cano and M. Tzanakakis for critical reading of the manuscript. Work in M. H.'s laboratory was supported by grants from the Juvenile Diabetes Research Foundation, the Hillblom Foundation and the National Institutes of Health.
Author information
Authors and Affiliations
Glossary
- HEPTAHELICAL BUNDLE
-
A transmembrane domain of the Smoothened protein that is composed of seven α-helical stretches.
- EXOCRINE ACIN
-
Alveolar structures that are formed by the cells that produce and release pancreatic digestive enzymes in the lumen of collecting pancreatic ducts.
Rights and permissions
About this article
Cite this article
di Magliano, M., Hebrok, M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer 3, 903–911 (2003). https://doi.org/10.1038/nrc1229
Issue Date:
DOI: https://doi.org/10.1038/nrc1229
This article is cited by
-
PRC2 mediated KLF2 down regulation: a therapeutic and diagnostic axis during tumor progression
Cancer Cell International (2023)
-
Functionally distinct cancer-associated fibroblast subpopulations establish a tumor promoting environment in squamous cell carcinoma
Nature Communications (2023)
-
Primary cilia on muscle stem cells are critical to maintain regenerative capacity and are lost during aging
Nature Communications (2022)
-
FOXA1 is a transcriptional activator of Odf2/Cenexin and regulates primary ciliation
Scientific Reports (2022)
-
Understanding Abnormal SMO-SHH Signaling in Autism Spectrum Disorder: Potential Drug Target and Therapeutic Goals
Cellular and Molecular Neurobiology (2022)