Key Points
-
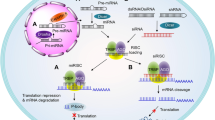
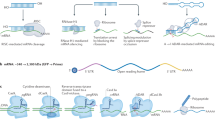
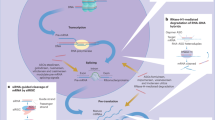
Many cancer targets are difficult to block with conventional therapies. Although RNA interference (RNAi) as a therapeutic approach is appealing, many challenges to delivery must be overcome.
-
Nanoparticles hold promise for the safe and effective intracellular delivery of RNAi-based molecules.
-
Physiological barriers and systemic toxicity of nanoparticle-based carrier systems create multiple challenges to bringing RNAi-based therapeutics to the clinic.
-
Nanoparticles can be used to help avoid immune-mediated responses to systemic RNAi-based therapy.
-
Solutions to improving tumour specificity and the ability to monitor and control short-term and long-term RNAi-based therapies are crucial next steps before clinical use.
-
As the technology for delivery improves, so we will also need to improve our understanding of the heterogeneity of RNAi processing in different cancer types.
-
Various resistance mechanisms to RNAi-based therapies must be anticipated.
Abstract
Inherent difficulties with blocking many desirable targets using conventional approaches have prompted many to consider using RNA interference (RNAi) as a therapeutic approach. Although exploitation of RNAi has immense potential as a cancer therapeutic, many physiological obstacles stand in the way of successful and efficient delivery. This Review explores current challenges to the development of synthetic RNAi-based therapies and considers new approaches to circumvent biological barriers, to avoid intolerable side effects and to achieve controlled and sustained release.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Fire, A. et al. Potent and specific genetic interference by double-strandedRNA in Caenorhabditis elegans. Nature 391, 806–811 (1998).
Druker, B. J. et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 355, 2408–2417 (2006).
Smith, I. et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet 369, 29–36 (2007).
Kikani, C. K., Dong, L. Q. & Liu, F. “New”-clear functions of PDK1: beyond a master kinase in the cytosol? J. Cell. Biochem. 96, 1157–1162 (2005).
Lim, S. T., Mikolon, D., Stupack, D. G. & Schlaepfer, D. D. FERM control of FAK function: implications for cancer therapy. Cell Cycle 7, 2306–2314 (2008).
Weihua, Z. et al. Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell 13, 385–393 (2008).
Davis, M. E. et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 464, 1067–1070 (2010). This is the first report of using targeted nanoparticles for siRNA delivery in humans.
Sashital, D. G. & Doudna, J. A. Structural insights into RNA interference. Curr. Opin. Struct. Biol. 20, 90–97 (2010).
Rana, T. M. Illuminating the silence: understanding the structure and function of small RNAs. Nature Rev. Mol. Cell Biol. 8, 23–36 (2007).
Heo, I. & Kim, V. N. Regulating the regulators: posttranslational modifications of RNA silencing factors. Cell 139, 28–31 (2009).
Hannon, G. J. & Rossi, J. J. Unlocking the potential of the human genome with RNA interference. Nature 431, 371–378 (2004).
Meister, G. & Tuschl, T. Mechanisms of gene silencing by double-stranded RNA. Nature 431, 343–349 (2004).
Martinez, J., Patkaniowska, A., Urlaub, H., Lührmann, R. & Tuschl, T. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell 110, 565–574 (2002).
Liu, J. et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science 305, 1437–1441 (2004).
Meister, G. et al. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell 15, 185–197 (2004).
Kim, V. N. MicroRNA biogenesis: coordinated cropping and dicing. Nature Rev. Mol. Cell Biol. 6, 376–385 (2005).
Bohnsack, M. T., Czaplinski, K. & Gorlich, D. Exportin-5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA 10, 185–191 (2004).
Chu, C. & Rana, T. M. Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54. PLoS Biol. 4, e210 (2006).
Fabbri, M. & Calin, G. A. Beyond genomics: interpreting the 93% of the human genome that does not encode proteins. Curr. Opin. Drug Discov. Devel. 13, 350–358.
Bumcrot, D., Manoharan, M., Koteliansky, V. & Sah, D. W. Y. RNAi therapeutics: a potential new class of pharmaceutical drugs. Nature Chem. Biol. 2, 711–719 (2006).
Soutschek, J. et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 432, 173–178 (2004).
Czauderna, F. et al. Structural variations and stabilizing modifications of synthetic siRNAs in mammalian cells. Nucleic Acids Res. 31, 2705–2716 (2003).
Kim, D. H. & Rossi, J. J. Strategies for silencing human disease using RNA interference. Nature Rev. Genet. 8, 173–184 (2007).
Dobrovolskaia, M. A., Aggarwal, P., Hall, J. B. & McNeil, S. E. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol. Pharm. 5, 487–495 (2008).
Skinner, S. A., Tutton, P. J. & O'Brien, P. E. Microvascular architecture of experimental colon tumors in the rat. Cancer Res. 50, 2411–2417 (1990).
Decuzzi, P., Causa, F., Ferrari, M. & Netti, P. A. The effective dispersion of nanovectors within the tumor microvasculature. Ann. Biomed. Eng. 34, 633–641 (2006).
Tong, R. T. et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 64, 3731–3736 (2004).
Matsumura, Y. & Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46, 6387–6392 (1986). This work describes the first observation of the EPR effect in the tumour microenvironment.
Maeda, H. Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjug. Chem. 21, 797–802 (2010).
Decuzzi, P. & Ferrari, M. The receptor-mediated endocytosis of nonspherical particles. Biophys. J. 94, 3790–3797 (2008).
Dominska, M. & Dykxhoorn, D. M. Breaking down the barriers: siRNA delivery and endosome escape. J. Cell Sci. 123, 1183–1189 (2010).
Kleeff, J. et al. Pancreatic cancer microenvironment. Int. J. Cancer 121, 699–705 (2007).
Burke, R. S. & Pun, S. H. Extracellular barriers to in Vivo PEI and PEGylated PEI polyplex-mediated gene delivery to the liver. Bioconjug. Chem. 19, 693–704 (2008).
Goodman, T. T., Ng, C. P. & Pun, S. H. 3-D tissue culture systems for the evaluation and optimization of nanoparticle-based drug carriers. Bioconjug. Chem. 19, 1951–1959 (2008).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1477–1478 (2004).
Bartneck, M., Keul, H. A., Zwadlo-Klarwasser, G. & Groll, J. Phagocytosis independent extracellular nanoparticle clearance by human immune cells. Nano Lett. 10, 59–63 (2010).
Judge, A. D. et al. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nature Biotech. 23, 457–462 (2005).
Forsbach, A. et al. Identification of RNA sequence motifs stimulating sequence-specific TLR8-dependent immune responses. J. Immunol. 180, 3729–3738 (2008).
Jackson, A. L. & Linsley, P. S. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nature Rev. Drug Discov. 9, 57–67 (2010).
Robbins, M., Judge, A. & MacLachlan, I. siRNA and innate immunity. Oligonucleotides 19, 89–101 (2009).
Kleinman, M. E. et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature 452, 591–597 (2008). This work shows that TLR3 activation by naked siRNAs can cause an anti-angiogenic off-target effect independently of gene silencing.
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nature Immunol. 5, 730–737 (2004).
Sakamoto, J. H. et al. Enabling individualized therapy through nanotechnology. Pharmacol. Res. 1–31 (2010).
Ma, Z. et al. Cationic lipids enhance siRNA-mediated interferon response in mice. Biochem. Biophys. Res. Commun. 330, 755–759 (2005).
Omidi, Y., Barar, J. & Akhtar, S. Toxicogenomics of cationic lipid-based vectors for gene therapy: impact of microarray technology. Curr. Drug Deliv. 2, 429–441 (2005).
Dokka, S., Toledo, D., Shi, X., Castranova, V. & Rojanasakul, Y. Oxygen radical-mediated pulmonary toxicity induced by some cationic liposomes. Pharm. Res. 17, 521–525 (2000).
Gutierrez-Puente, Y. et al. Cellular pharmacology of P-ethoxy antisense oligonucleotides targeted to Bcl-2 in a follicular lymphoma cell line. Leuk. Lymphoma 44, 1979–1985 (2003).
Landen, C. N. et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 65, 6910–6918 (2005).
Ahmed, A. A. et al. SIK2 is a centrosome kinase required for bipolar mitotic spindle formation that provides a potential target for therapy in ovarian cancer. Cancer Cell 18, 109–121 (2010).
Schroeder, A., Levins, C. G., Cortez, C., Langer, R. & Anderson, D. G. Lipid-based nanotherapeutics for siRNA delivery. J. Intern. Med. 267, 9–21 (2010).
Kim, S. H., Jeong, J. H., Lee, S. H., Kim, S. W. & Park, T. G. Local and systemic delivery of VEGF siRNA using polyelectrolyte complex micelles for effective treatment of cancer. J. Control. Release 129, 107–116 (2008).
Wolfrum, C. et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nature Biotech. 25, 1149–1157 (2007).
Akinc, A. et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nature Biotech. 26, 561–569 (2008).
Love, K. T. et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc. Natl Acad. Sci. USA 107, 1864–1869 (2010).
Hunter, A. C. Molecular hurdles in polyfectin design and mechanistic background to polycation induced cytotoxicity. Adv. Drug Deliv. Rev. 58, 1523–1531 (2006).
Jain, K., Kesharwani, P., Gupta, U. & Jain, N. K. Dendrimer toxicity: let's meet the challenge. Int. J. Pharm. 394, 122–142 (2010).
Pille, J. Y. et al. Intravenous delivery of anti-RhoA small interfering RNA Loaded in nanoparticles of chitosan in mice: safety and efficacy in xenografted aggressive breast cancer. Hum. Gene Ther. 17, 1019–1026 (2006).
Han, H. D. et al. Targeted gene silencing using RGD-labeled chitosan nanoparticles. Clin. Cancer Res. 16, 3910–3922 (2010).
Lu, C. et al. Regulation of tumor angiogenesis by EZH2. Cancer Cell 18, 185–197 (2010).
Heidel, J. D. et al. Administration in non-human primates of escalating intravenous doses of targeted nanoparticles containing ribonucleotide reductase subunit M2 siRNA. Proc. Natl Acad. Sci. USA 104, 5715–5721 (2007).
Takeshita, F. et al. Efficient delivery of small interfering RNA to bone-metastatic tumors by using atelocollagen in vivo. Proc. Natl Acad. Sci. USA 102, 12177–12182 (2005).
Jackson, A. L. et al. Expression profiling reveals off-target gene regulation by RNAi. Nature Biotech. 21, 635–637 (2003). This study demonstrated that siRNAs can cause miRNA-like translational suppression owing to imperfect complementarity in the seed sequence.
Jackson, A. L. et al. Widespread siRNA “off-target” transcript silencing mediated by seed region sequence complementarity. RNA 12, 1179–1187 (2006).
Farh, K. K. et al. The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science 310, 1817–1821 (2005).
Jackson, A. L. et al. Position-specific chemical modification of siRNAs reduces “off-target” transcript silencing. RNA 12, 1197–1205 (2006).
Merritt, W. M. et al. Dicer, Drosha, and outcomes in patients with ovarian cancer. N. Engl. J. Med. 359, 2641–2650 (2008). This study shows changes in Dicer and Drosha levels in cancer have effects on gene silencing.
Grimm, D. et al. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature 441, 537–541 (2006).
Castanotto, D. et al. Combinatorial delivery of small interfering RNAs reduces RNAi efficacy by selective incorporation into RISC. Nucleic Acids Res. 35, 5154–5164 (2007).
Khan, A. A. et al. Transfection of small RNAs globally perturbs gene regulation by endogenous microRNAs. Nature Biotech. 27, 549–555 (2009). This paper demonstrates how exogenous introduction of RNAi can compete with endogenous miRNAs, causing derepression of miRNA-regulated genes.
Haley, B. & Zamore, P. D. Kinetic analysis of the RNAi enzyme complex. Nature Struct. Mol. Biol. 11, 599–606 (2004).
Raemdonck, K., Vandenbroucke, R. E., Demeester, J., Sanders, N. N. & De Smedt, S. C. Maintaining the silence: reflections on long-term RNAi. Drug Discov. Today 13, 917–931 (2008).
Takabatake, Y. et al. Chemically modified siRNA prolonged RNA interference in renal disease. Biochem. Biophys. Res. Commun. 363, 432–437 (2007).
Bartlett, D. W. & Davis, M. E. Insights into the kinetics of siRNA-mediated gene silencing from live-cell and live-animal bioluminescent imaging. Nucleic Acids Res. 31, 322–333 (2006).
Duncan, R. The dawning era of polymer therapeutics. Nature Rev. Drug Discov. 2, 347–360 (2003).
Shahzad, M. M. K. et al. Dual targeting of EphA2 and FAK in ovarian carcinoma. Cancer Biol. Ther. 8, 1027–1034 (2009).
Hogrefe, R. I. et al. Chemically modified short interfering hybrids (siHYBRIDS): nanoimmunoliposome delivery in vitro and in vivo for RNAi of HER-2. Nucleosides Nucleotides Nucleic Acids 25, 889–907 (2006).
Ferrari, M. Frontiers in cancer nanomedicine: directing mass transport through biological barriers. Trends Biotech. 28, 181–188 (2010).
Estévez, M. C. et al. Nanoparticle-aptamer conjugates for cancer cell targeting and detection. Methods Mol. Biol. 624, 235–248 (2010).
Kim, S. H., Mok, H., Jeong, J. H., Kim, S. W. & Park, T. G. Comparative evaluation of target-specific GFP gene silencing efficiencies for antisense ODN, synthetic siRNA, and siRNA plasmid complexed with PEI-PEG-FOL conjugate. Bioconjug. Chem. 17, 241–244 (2006).
Passarella, R. J. et al. Targeted nanoparticles that deliver a sustained, specific release of paclitaxel to irradiated tumors. Cancer Res. 70, 4550–4559 (2010).
Tanaka, T. et al. Sustained small interfering RNA delivery by mesoporous silicon particles. Cancer Res. 70, 3687–3696 (2010). This work demonstrates the first in vivo validation of multi-staged siRNA delivery for sustained gene silencing.
Decuzzi, P. & Ferrari, M. Design maps for nanoparticles targeting the diseased microvasculature. Biomaterials 29, 377–384 (2008).
Ferrari, M. The mathematical engines of nanomedicine. Small 4, 20–25 (2008).
Sanga, S. et al. Mathematical modeling of cancer progression and response to chemotherapy. Expert Rev. Anticancer Ther. 6, 1361–1376 (2006).
Moghimi, S. M., Hunter, A. C. & Murray, J. C. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol. Rev. 53, 283–318 (2001).
Alexis, F., Pridgen, E., Molnar, L. K. & Farokhzad, O. C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 5, 505–515 (2008).
Thall, P. F. & Cook, J. D. Dose-finding based on efficacy-toxicity trade-offs. Biometrics 60, 684–693 (2004).
Bruchez, M. J., Moronne, M., Gin, P., Weiss, S. & Alivisatos, A. P. Semiconductor nanocrystals as fluorescent biological labels. Science 281, 2013–2016 (1998).
Tan, W. B., Jiang, S. & Zhang, Y. Quantum-dot based nanoparticles for targeted silencing of HER2/neu gene via RNA interference. Biomaterials 28, 1565–1571 (2007).
Tavares, A. J., Chong, L., Petryayeva, E., Algar, W. R. & Krull, U. J. Quantum dots as contrast agents for in vivo tumor imaging: progress and issues. Anal. Bioanal. Chem. 25 Jul 2010 (doi:10.1007/s00216-010-4010-3).
Hong, H., Zhang, Y. & Cai, W. In vivo imaging of RNA interference. J. Nucl. Med. 51, 169–172 (2010).
Chen, X. Q. et al. Telomerase RNA as a detection marker in the serum of breast cancer patients. Clin. Cancer Res. 6, 3823–3826 (2000).
Kopreski, M. S., Benko, F. A., Kwak, L. W. & Gocke, C. D. Detection of tumor messenger RNA in the serum of patients with malignant melanoma. Clin. Cancer Res. 5, 1961–1965 (1999).
Swarup, V. & Rajeswari, M. R. Circulating (cell-free) nucleic acids – a promising, non-invasive tool for early detection of several human diseases. FEBS J. 581, 795–799 (2007).
Abdrakhmanova, A. et al. RNAi and iTRAQ reagents united: targeted quantitation of siRNA-mediated protein silencing in human cells. Anal. Bioanal. Chem. 395, 773–785 (2009).
Spielman, R. S. et al. Common genetic variants account for differences in gene expression among ethnic groups. Nature Genet. 39, 226–231 (2007).
Nicoloso, M. S. et al. Single-nucleotide polymorphisms inside microRNA target sites influence tumor susceptibility. Cancer Res. 70, 2789–2798 (2010).
Merritt, W. M., Bar-Eli, M. & Sood, A. K. The dicey role of Dicer: implications for RNAi therapy. Cancer Res. 70, 2571–2574 (2010).
Diederichs, S. et al. Coexpression of Argonaute-2 enhances RNA interference toward perfect match binding sites. Proc. Natl Acad. Sci. USA 105, 9284–9289 (2008).
Acknowledgements
Portions of this work were supported by the NIH (CA110793, CA109298, P50 CA083639, P50 CA098258, CA128797, RC2GM092599 and U54 CA151668), the Ovarian Cancer Research Fund, Inc. (Program Project Development Grant), the DOD (OC073399, W81XWH-10-1-0158 and BC085265), the Zarrow Foundation, the Laura and John Arnold Foundation and the Betty Anne Asche Murray Distinguished Professorship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- RNA interference
-
(RNAi). Refers to the mechanism of potent and specific gene-silencing caused by double-stranded RNA (dsRNA) produced by endogenous (miRNA) or exogenous (siRNA) sources.
- Opsonins
-
Plasma proteins that act as binding enhancers for phagocytosis.
- Reticuloendothelial system
-
(RES). System composed of scavenging monocytes and macrophages located in the reticular connective tissue (notably the liver, spleen, lung and marrow).
- Enhanced permeability and retention
-
(EPR). The property by which certain sizes of molecules tend to accumulate and remain in tumour tissue more than in normal tissues.
- Endocytosis
-
The active uptake of molecules into a cell by clathrin-dependent and clathrin-independent receptor-mediated endocytosis, pinocytosis and phagocytosis.
- Fusogenic lipids
-
Lipoplexes (containing cationic lipids and nucleic acids) that adopt an inverted hexagonal phase and fuse with anionic membranes resulting in endosomal release.
- Fusogenic peptides
-
Peptides that have cell penetrating properties (such as being highly hydrophobic), which cause cell membrane destabilization and intra-cytoplasmic release.
- pH-sensitive lipoplexes
-
Liposomes that hydrolyse and trigger release of contents owing to subtle drops in pH, such as with endosomal fusion, allowing for endosomal escape.
- pH-sensitive polyplexes
-
Polyplexes (cationic polymer complexed with nucleic acid) that act as proton 'sponges', preventing acidification after endosomal fusion and allowing influx of counter ions, osmotic swelling and endosome rupture.
- Aptamer
-
A DNA or RNA oligonucleotide sequence with a high-affinity binding for specific proteins.
- Logic-embedded vector
-
Vehicles that work in a time-sequential manner to cross biological barriers.
- Mesoporous silicon
-
A biodegradable and biocompatible material made from non-oxidized silicon.
- Polyethylene glycol
-
(PEG). A synthetic polymer that is non-toxic, non-immunogenic and highly water soluble.
- Quantum dots
-
Colloidal semiconductor nanocrystals with optical and electronic properties superior to conventional organic fluorophores that can be used for imaging purposes.
Rights and permissions
About this article
Cite this article
Pecot, C., Calin, G., Coleman, R. et al. RNA interference in the clinic: challenges and future directions. Nat Rev Cancer 11, 59–67 (2011). https://doi.org/10.1038/nrc2966
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc2966
This article is cited by
-
Biotechnological strategies to decipher the functions of abiotic stress-associated genes in soybean
Plant Biotechnology Reports (2024)
-
Biomembrane-derived nanoplexes for SiRNAs-pioneer innovation in delivery to lung adenocarcinoma
Journal of Nanoparticle Research (2024)
-
Advances in functional lipid nanoparticles: from drug delivery platforms to clinical applications
3 Biotech (2024)
-
Biodegradable FePS3 nanoplatform for efficient treatment of osteosarcoma by combination of gene and NIR-II photothermal therapy
Journal of Nanobiotechnology (2023)
-
Nano-immunotherapy: overcoming delivery challenge of immune checkpoint therapy
Journal of Nanobiotechnology (2023)