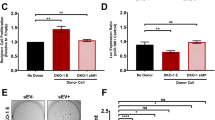
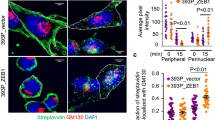
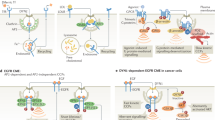
Abstract
Epithelial cell carcinogenesis involves the loss of cell polarity, alteration of polarized protein presentation, dynamic cell morphology changes, increased proliferation, and increased cell motility and invasion. Membrane vesicle trafficking underlies all of these processes. Specific membrane trafficking regulators, including RAB small GTPases, through the coordinated dynamics of intracellular trafficking along cytoskeletal pathways, determine the cell surface presentation of proteins and the overall function of both differentiated and neoplastic cells. Although mutations in vesicle trafficking proteins may not be direct drivers of transformation, components of the machinery of vesicle movement have crucial roles in the phenotypes of neoplastic cells. Therefore, the regulators of membrane vesicle trafficking decisions are essential mediators of the full range of cell physiologies that drive cancer cell biology, including initial loss of cell polarity, invasion and metastasis. Targeting of these fundamental intracellular processes may permit the manipulation of cancer cell behaviour.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mellman, I. & Nelson, W. J. Coordinated protein sorting, targeting and distribution in polarized cells. Nature Rev. Mol. Cell Biol. 9, 833–845 (2008).
Marrs, J. A. et al. Plasticity in epithelial cell phenotype: modulation by expression of different cadherin cell adhesion molecules. J. Cell Biol. 129, 507–519 (1995).
Rodriguez-Boulan, E. & Powell, S. K. Polarity of epithelial and neuronal cells. Ann. Rev. Cell Biol. 8, 395–427 (1992).
Royer, C. & Lu, X. Epithelial cell polarity: a major gatekeeper against cancer? Cell Death Differ. 18, 1470–1477 (2011).
McCaffrey, L. M. & Macara, I. G. Epithelial organization, cell polarity and tumorigenesis. Trends Cell Biol. 21, 727–735 (2011).
Dozynkiewicz, M. A. et al. Rab25 and CLIC3 collaborate to promote integrin recycling from late endosomes/lysosomes and drive cancer progression. Dev. Cell 22, 131–145 (2012).
Tang, B. L. & Ng, E. L. Rabs and cancer cell motility. Cell. Motil. Cytoskeleton 66, 365–370 (2009).
Krishnan, M., Lapierre, L. A., Knowles, B. C. & Goldenring, J. R. Rab25 regulates intergrin expression and trafficking in polarized colonic epithelial cells. Mol. Biol. Cell 24, 818–831 (2013).
Nelson, W. J. et al. Regulation of epithelial cell polarity: a view from the cellsurface. Cold Spring Harb. Symp. Quant. Biol. 57, 621–630 (1992).
Yeaman, C., Grindstaff, K. K. & Nelson, W. J. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol. Rev. 79, 73–98 (1999).
Fuller, S. D. & Simons, K. Transferrin receptor polarity and recycling accuracy in 'tight' and 'leaky' strains of Madin-Darby canine kidney cells. J. Cell Biol. 103, 1767–1779 (1986).
Wang, E. et al. Apical and basolateral endocytic pathways of MDCK cells meet in acidic common endosomes distinct from a nearly neutral apical recycling endosome. Traffic 1, 480–493 (2000).
Kostaras, E. et al. SARA and RNF11 interact with each other and ESCRT-0 core proteins and regulate degradative EGFR trafficking. Oncogene http://dx.doi.org/10.1038/onc.2012.554(2012).
Balaji, K. et al. RIN1 orchestrates the activation of RAB5 GTPases and ABL tyrosine kinases to determine the fate of EGFR. J. Cell Sci. 125, 5887–5896 (2012).
Goh, L. K., Huang, F., Kim, W., Gygi, S. & Sorkin, A. Multiple mechanisms collectively regulate clathrin-mediated endocytosis of the epidermal growth factor receptor. J. Cell Biol. 189, 871–883 (2010).
Derby, M. C. et al. The trans-Golgi network golgin, GCC185, is required for endosome-to-Golgi transport and maintenance of Golgi structure. Traffic 8, 758–773 (2007).
Tzaban, S. et al. The recycling and transcytotic pathways for IgG transport by FcRn are distinct and display an inherent polarity. J. Cell Biol. 185, 673–684 (2009).
Apodaca, G., Katz, L. A. & Mostov, K. E. Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J. Cell Biol. 125, 67–86 (1994).
Casanova, J. E. et al. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol. Biol. Cell 10, 47–61 (1999).
Erickson, R. P., Larson-Thome, K., Valenzuela, R. K., Whitaker, S. E. & Shub, M. D. Navajo microvillous inclusion disease is due to a mutation in MYO5B. Am. J. Med. Genet. A 146A, 3117–3119 (2008).
Muller, T. et al. MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nature Genet. 40, 1163–1165 (2008).
Feng, S. et al. A Rab8 guanine nucleotide exchange factor-effector interaction network regulates primary ciliogenesis. J. Biol. Chem. 287, 15602–15609 (2012).
Nachury, M. V. et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129, 1201–1213 (2007).
Schwartz, S. L., Cao, C., Pylypenko, O., Rak, A. & Wandinger-Ness, A. Rab GTPases at a glance. J. Cell Sci. 120, 3905–3910 (2007).
Kloer, D. P. et al. Assembly of the biogenesis of lysosome-related organelles complex-3 (BLOC-3) and its interaction with Rab9. J. Biol. Chem. 285, 7794–7804 (2010).
Wu, X., Sakamoto, T., Zhang, F., Sellers, J. R. & Hammer, J. A. 3rd. In vitro reconstitution of a transport complex containing Rab27a, melanophilin and myosin Va. FEBS Lett. 580, 5863–5868 (2006).
Jou, T.-Z. & Nelson, J. W. Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity. J. Cell Biol. 142, 85–100 (1998).
Bourne, H. Do GTPases direct membrane traffic in secretion? Cell 53, 669–671 (1988).
Bourne, H. R., Sanders, D. A. & McCormick, F. The GTPase superfamily: conserved structure and molecular mechanism. Nature 349, 117–127 (1991).
Frasa, M. A., Koessmeier, K. T., Ahmadian, M. R. & Braga, V. M. Illuminating the functional and structural repertoire of human TBC/RABGAPs. Nature Rev. Mol. Cell Biol. 13, 67–73 (2012).
Kraus, M. H., Yuasa, Y. & Aaronson, S. A. A position 12-activated H-ras oncogene in all HS578T mammary carcinosarcoma cells but not normal mammary cells of the same patient. Proc. Natl Acad. Sci. USA 81, 5384–5388 (1984).
Eva, A., Tronick, S. R., Gol, R. A., Pierce, J. H. & Aaronson, S. A. Transforming genes of human hematopoietic tumors: frequent detection of ras-related oncogenes whose activation appears to be independent of tumor phenotype. Proc. Natl Acad. Sci. USA 80, 4926–4930 (1983).
Hales, C. M. et al. Identification and characterization of a family of Rab11-interacting proteins. J. Biol. Chem. 276, 39067–39075 (2001).
Prekeris, R., Davies, J. M. & Scheller, R. H. Identificaition of a novel Rab11/25 binding domain in eferin and Rip proteins. J. Biol. Chem. 276, 38966–38970 (2001).
Mammoto, A., Ohtsuka, T., Hotta, I., Sasaki, T. & Takai, Y. Rab11BP/Rabphillin-11, a downstream target of Rab11 small G protein implicated in vesicle recycling. J. Biol. Chem. 274, 25517–25524 (1999).
Lapierre, L. A. et al. Myosin vb is associated with plasma membrane recycling systems. Mol. Biol. Cell 12, 1843–1857 (2001).
Roland, J. T., Lapierre, L. A. & Goldenring, J. R. Alternative splicing in class V myosins determines association with Rab10. J. Biol. Chem. 284, 1213–1223 (2009).
Knodler, A. et al. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc. Natl Acad. Sci. USA 107, 6346–6351 (2010).
Wu, S., Mehta, S. Q., Pichaud, F., Bellen, H. J. & Quiocho, F. A. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nature Struct. Mol. Biol. 12, 879–885 (2005).
Zhang, X. M., Ellis, S. Sriratana, A., Mitchell, C. A. & Rowe, T. Sec15 is an effector for the Rab11 GTPase in mammalian cells. J. Biol. Chem. 279, 43027–43034 (2004).
Takahashi, S. et al. Rab11 regulates exocytosis of recycling vesicles at the plasma membrane. J. Cell Sci. 125, 4049–4057 (2012).
Oztan, A. et al. Exocyst requirement for endocytic traffic directed toward the apical and basolateral poles of polarized MDCK cells. Mol. Biol. Cell 18, 3978–3992 (2007).
Jin, M. & Goldenring, J. R. The Rab11-FIP1/RCP gene codes for multiple protein transcripts related to the plasma membrane recycling system. Biochim. Biophys. Acta 1759, 281–295 (2006).
Baetz, N. W. & Goldenring, J. R. Rab11-family interacting proteins define spatially and temporally distinct regions within the dynamic Rab11a-dependent recycling system. Mol. Biol. Cell 24, 643–658 (2013).
Vitale, G. et al. Distinct Rab-binding domains mediate the interaction of Rabaptin-5 with GTP-bound rab4 and rab5. EMBO J. 17, 1941–1951 (1998).
Qi, M. et al. Rab11-FIP1C and Rab14 direct plasma membrane sorting and particle incorporation of the HIV-1 envelope glycoprotein complex. PLoS Pathog. 9, e1003278 (2013).
Kelly, E. E. et al. Class I Rab11-family interacting proteins are binding targets for the Rab14 GTPase. Biol. Cell 102, 51–62 (2010).
Roland, J. T., Kenworthy, A. K., Peranen, J., Caplan, S. & Goldenring, J. R. Myosin Vb interacts with Rab8a on a tubular network containing EHD1 and EHD3. Mol. Biol. Cell 18, 2828–2837 (2007).
Strom, M., Hume, A. N., Tarafder, A. K., Barkagianni, E. & Seabra, M. C. A family of Rab27a-binding proteins: melanophilin links Rab27a and myosin Va functions in melanosome transport. J. Biol. Chem. 277, 25423–25430 (2002).
Ramalho, J. S., Lopes, V. S., Tarafder, A. K., Seabra, M. C. & Hume, A. N. Myrip uses distinct domains in the cellular activation of myosin VA and myosin VIIA in melanosome transport. Pigment Cell. Melanoma Res. 22, 461–473 (2009).
Fielding, A. B. et al. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J. 24, 3389–3399 (2005).
Hickson, G. R. et al. Arfophilins are dual Arf/Rab 11 binding proteins that regulate recycling endosome distribution and are related to Drosophila nuclear fallout. Mol. Biol. Cell 14, 2908–2920 (2003).
Cresawn, K. O. et al. Differential involvement of endocytic compartments in the biosynthetic traffic of apical proteins. EMBO J. 26, 3737–3748 (2007).
Capilla, A. et al. Planar cell polarity controls directional Notch signaling in the Drosophila leg. Development 139, 2584–2593 (2012).
Mateus, A. M., Gorfinkiel, N., Schamberg, S. & Martinez Arias, A. Endocytic and recycling endosomes modulate cell shape changes and tissue behaviour during morphogenesis in Drosophila. PLoS ONE 6, e18729 (2011).
Roeth, J. F., Sawyer, J. K., Wilner, D. A. & Peifer, M. Rab11 helps maintain apical crumbs and adherens junctions in the Drosophila embryonic ectoderm. PLoS ONE 4, e7634 (2009).
Lu, H. & Bilder, D. Endocytic control of epithelial polarity and proliferation in Drosophila. Nature Cell Biol. 7, 1232–1239 (2005).
Morrison, H. A. et al. Regulation of early endosomal entry by the Drosophila tumor suppressors Rabenosyn and Vps45. Mol. Biol. Cell 19, 4167–4176 (2008).
Guilford, P. et al. E-cadherin germline mutations in familial gastric cancer. Nature 392, 402–405 (1998).
Oshima, M. et al. Loss of Apc heterozygosity and abnormal tissue building in nascent intestinal polyps in mice carrying a truncated Apc gene. Proc. Natl Acad. Sci. USA 92, 4482–4486 (1995).
Bertwistle, D. & Ashworth, A. The pathology of familial breast cancer: How do the functions of BRCA1 and BRCA2 relate to breast tumour pathology? Breast Cancer Res. 1, 41–47 (1999).
Jones, R. G. et al. Conditional deletion of beta1 integrins in the intestinal epithelium causes a loss of Hedgehog expression, intestinal hyperplasia, and early postnatal lethality. J. Cell Biol. 175, 505–514 (2006).
Nam, K. T. et al. Loss of Rab25 promotes the development of intestinal neoplasia in mice and is associated with human colorectal adenocarcinomas. J. Clin. Invest. 120, 840–849 (2010).
Gephart, J. D. et al. Identification of a novel mono-leucine basolateral sorting motif within the cytoplasmic domain of amphiregulin. Traffic 12, 1793–1804 (2011).
Singh, B., Bogatcheva, G., Washington, M. K. & Coffey, R. J. Transformation of polarized epithelial cells by apical mistrafficking of epiregulin. Proc. Natl Acad. Sci. USA 110, 8960–8965 (2013).
Kuwada, S. K. & Li, X. Integrin α5/β1 mediates fibronectin-dependent epithelial cell proliferation through epidermal growth factor receptor activation. Mol. Biol. Cell 11, 2485–2496 (2000).
Muller, P. A. et al. Mutant p53 drives invasion by promoting integrin recycling. Cell 139, 1327–1341 (2009).
Rainero, E. et al. Diacylglycerol kinase alpha controls RCP-dependent integrin trafficking to promote invasive migration. J. Cell Biol. 196, 277–295 (2012).
Arjonen, A., Alanko, J., Veltel, S. & Ivaska, J. Distinct recycling of active and inactive β1 Integrins. Traffic 13, 610–625 (2012).
Hayashi, D. et al. Deficiency of claudin-18 causes paracellular H+ leakage, up-regulation of interleukin-1β, and atrophic gastritis in mice. Gastroenterology 142, 292–304 (2012).
Ivanov, A. I., Nusrat, A. & Parkos, C. A. The epithelium in inflammatory bowel disease: potential role of endocytosis of junctional proteins in barrier disruption. Novartis Found. Symp. 263, 115–124 (2004).
Lioni, M. et al. Dysregulation of claudin-7 leads to loss of E-cadherin expression and the increased invasion of esophageal squamous cell carcinoma cells. Am. J. Pathol. 170, 709–721 (2007).
Williams, K. C. & Coppolino, M. G. Phosphorylation of membrane type 1-matrix metalloproteinase (MT1-MMP) and its vesicle-associated membrane protein 7 (VAMP7)-dependent trafficking facilitate cell invasion and migration. J. Biol. Chem. 286, 43405–43416 (2011).
Yin, X., Murphy, S. J., Wilkes, M. C., Ji, Y. & Leof, E. B. Retromer maintains basolateral distribution of the type II TGFβ receptor via the recycling endosome. Mol. Biol. Cell 24, 2285–2298 (2013).
Tringali, C. et al. The plasma membrane sialidase NEU3 regulates the malignancy of renal carcinoma cells by controlling beta1 integrin internalization and recycling. J. Biol. Chem. 287, 42835–42845 (2012).
Cheng, J. M. et al. Tumor suppressor function of Rab25 in triple-negative breast cancer. Int. J. Cancer 126, 2799–2812 (2010).
Amornphimoltham, P. et al. Rab25 regulates invasion and metastasis in head and neck cancer. Clin. Cancer Res. 19, 1375–1388 (2013).
Cheng, K. W. et al. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nature Med. 10, 1251–1256 (2004).
Caswell, P. T. et al. Rab25 associates with α5β1 integrin to promote invasive migration in 3D microenvironments. Dev. Cell 13, 496–510 (2007).
Faber, A. et al. SDF-1-CXCR4 axis: cell trafficking in the cancer stem cell niche of head and neck squamous cell carcinoma. Oncol. Rep. 29, 2325–2331 (2013).
Ebner, R. & Derynck, R. Epidermal growth factor and transforming growth factor-a:differential intracellular routing and processing of ligand-receptor complexes. Cell Reg. 2, 599–612 (1991).
Lobert, V. H. et al. Ubiquitination of α5β1 integrin controls fibroblast migration through lysosomal degradation of fibronectin-integrin complexes. Dev. Cell 19, 148–159 (2010).
Chamberlain, M. D. et al. Disrupted RabGAP function of the p85 subunit of phosphatidylinositol 3-kinase results in cell transformation. J. Biol. Chem. 283, 15861–15868 (2008).
Allaire, P. D. et al. Interplay between Rab35 and Arf6 controls cargo recycling to coordinate cell adhesion and recycling. J. Cell Sci. 126, 722–731 (2012).
Hattula, K. et al. Characterization of the Rab8-specific membrane traffic route linked to protrusion formation. J. Cell Sci. 119, 4866–4877 (2006).
Kerber, M. L. et al. A novel form of motility in filopodia revealed by imaging myosin-X at the single-molecule level. Curr. Biol. 19, 967–973 (2009).
Pellinen, T. et al. Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of β1-integrins. J. Cell Biol. 173, 767–780 (2006).
Mai, A. et al. Competitive binding of Rab21 and p120RasGAP to integrins regulates receptor traffic and migration. J. Cell Biol. 194, 291–306 (2011).
Zhang, J. et al. RCP is a human breast cancer-promoting gene with Ras-activating function. J. Clin. Invest. 119, 2171–2183 (2009).
Hognas, G. et al. Cytokinesis failure due to derailed integrin traffic induces aneuploidy and oncogenic transformation in vitro and in vivo. Oncogene 31, 3597–3606 (2012).
Wilson, G. M. et al. The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol. Biol. Cell 16, 849–860 (2005).
Peranen, J. Rab8 GTPase as a regulator of cell shape. Cytoskeleton (Hoboken) 68, 527–539 (2011).
Beaumont, K. A. et al. The recycling endosome protein Rab17 regulates melanocytic filopodia formation and melanosome trafficking. Traffic 12, 627–643 (2011).
Barbarin, A. & Frade, R. Procathepsin L secretion, which triggers tumour progression, is regulated by Rab4a in human melanoma cells. Biochem. J. 437, 97–107 (2011).
Bravo-Cordero, J. J. et al. MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. EMBO J. 26, 1499–1510 (2007).
Steffen, A. et al. MT1-MMP-dependent invasion is regulated by TI-VAMP/VAMP7. Curr. Biol. 18, 926–931 (2008).
Poincloux, R., Lizarraga, F. & Chavrier, P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J. Cell Sci. 122, 3015–3024 (2009).
Itoh, Y. et al. Dimerization of MT1-MMP during cellular invasion detected by fluorescence resonance energy transfer. Biochem. J. 440, 319–326 (2011).
Hu, J. et al. Cdc42-interacting protein 4 is a Src substrate that regulates invadopodia and invasiveness of breast tumors by promoting MT1-MMP endocytosis. J. Cell Sci. 124, 1739–1751 (2011).
Fisher, K. E. et al. MT1-MMP- and Cdc42-dependent signaling co-regulate cell invasion and tunnel formation in 3D collagen matrices. J. Cell Sci. 122, 4558–4569 (2009).
Barker, N. et al. Very long-term self-renewal of small intestine, colon, and hair follicles from cycling Lgr5+ve stem cells. Cold Spring Harb. Symp. Quant. Biol. 73, 351–356 (2008).
Powell, A. E. et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 149, 146–158 (2012).
Sakamori, R. et al. Cdc42 and Rab8a are critical for intestinal stem cell division, survival, and differentiation in mice. J. Clin. Invest. 122, 1052–1065 (2012).
Shimada, K. et al. Aberrant expression of RAB1A in human tongue cancer. Br. J. Cancer 92, 1915–1921 (2005).
Yang, P. S. et al. Rab5A is associated with axillary lymph node metastasis in breast cancer patients. Cancer Sci. 102, 2172–2178 (2011).
Nakano, T. et al. Establishment of a human lung cancer cell line with high metastatic potential to multiple organs: gene expression associated with metastatic potential in human lung cancer. Oncol. Rep. 28, 1727–1735 (2012).
Wang, R. et al. MicroRNA-451 functions as a tumor suppressor in human non-small cell lung cancer by targeting ras-related protein 14 (RAB14). Oncogene 30, 2644–2658 (2011).
Ho, J. R. et al. Deregulation of Rab and Rab effector genes in bladder cancer. PLoS ONE 7, e39469 (2012).
Hou, Q. et al. Integrative genomics identifies RAB23 as an invasion mediator gene in diffuse-type gastric cancer. Cancer Res. 68, 4623–4630 (2008).
Cheng, J. M., Ding, M., Aribi, A., Shah, P. & Rao, K. Loss of RAB25 expression in breast cancer. Int. J. Cancer 118, 2957–2964 (2006).
Yin, Y. X. et al. Increased expression of Rab25 in breast cancer correlates with lymphatic metastasis. Tumour Biol. 33, 1581–1587 (2012).
Jacob, A. et al. Rab40b regulates MMP2 and MMP9 trafficking during invadopodia formation and breast cancer cell invasion. J. Cell Sci. http:dx.doi.org/10.1242/jcs.126573 (2013).
Acknowledgements
J.R.G.'s work is supported by the US National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants RO1 DK048370 and RO1 DK070856 and a VA Merit Review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Glossary
- Adherens junction
-
Intercellular adhesion structures that seal the lateral spaces between cells in simple epithelia and serve as a reference point for basolateral trafficking. They contain the intercellular adhesion molecules E-cadherin and p120 catenin.
- Apico–basal polarity
-
Separation in polarized epithelial cells between apical membrane surfaces that face the external environment and the basolateral membrane, which faces the internal milieu.
- Autophagosomes
-
Specialized lysosomal vacuoles that are responsible for degradation of intracellular organelles and recycling of components for use in de novo synthesis.
- Exocyst
-
An evolutionarily conserved (from yeast to humans) multiprotein complex that mediates exocytosis at the plasma membrane.
- Glycosylated
-
Pertaining to the addition of sugar residues to the external regions of membrane proteins.
- Microvilli
-
Organized plasma membrane protrusions on the apical surface of cells that increase the surface area and facilitate absorption and secretion.
- Primary cilia
-
In mammalian cells, a specialized protrusion with sensory functions.
- Resting short circuit current
-
The electrical manifestation of epithelial polarity manifested by junctional characteristics and directed ion pumps and channels.
- TBC domains
-
TBC (TRE2-BUB2-CDC16) domains are conserved motifs that are present in many RAB-GTPase activating proteins.
- Tight junction
-
Intercellular junctions that are composed of proteins such as ZO1, occludin and claudins and are responsible for the tightness of the barrier between epithelial cells.
- Tubulation of the recycling system
-
Part of a range of intracellular morphologies between small vesicles and tubules that indicates the process of trafficking dynamics.
Rights and permissions
About this article
Cite this article
Goldenring, J. A central role for vesicle trafficking in epithelial neoplasia: intracellular highways to carcinogenesis. Nat Rev Cancer 13, 813–820 (2013). https://doi.org/10.1038/nrc3601
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3601
This article is cited by
-
CircRTN4 promotes pancreatic cancer progression through a novel CircRNA-miRNA-lncRNA pathway and stabilizing epithelial-mesenchymal transition protein
Molecular Cancer (2022)
-
Myosin Vb as a tumor suppressor gene in intestinal cancer
Oncogene (2022)
-
Vacuolin-1 inhibits endosomal trafficking and metastasis via CapZβ
Oncogene (2021)
-
SCAMP2/5 as diagnostic and prognostic markers for acute myeloid leukemia
Scientific Reports (2021)
-
Cytoskeletal Organization and Cell Polarity in the Pathogenesis of Crohn’s Disease
Clinical Reviews in Allergy & Immunology (2021)