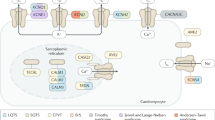
Abstract
Regulation of calcium flux in the heart is a key process that affects cardiac excitability and contractility. Degenerative diseases, such as coronary artery disease, have long been recognized to alter the physiology of intracellular calcium regulation, leading to contractile dysfunction or arrhythmias. Since the discovery of the first gene mutation associated with catecholaminergic polymorphic ventricular tachycardia (CPVT) in 2001, a new area of interest in this field has emerged—the genetic abnormalities of key components of the calcium regulatory system. Such anomalies cause a variety of genetic diseases characterized by the development of life-threatening arrhythmias in young individuals. In this Review, we provide an overview of the structural organization and the function of calcium-handling proteins and describe the mechanisms by which mutations determine the clinical phenotype. Firstly, we discuss mutations in the genes encoding the ryanodine receptor 2 (RYR2) and calsequestrin 2 (CASQ2). These proteins are pivotal to the regulation of calcium release from the sarcoplasmic reticulum, and mutations can cause CPVT. Secondly, we review defects in genes encoding proteins that form the voltage-dependent L-type calcium channel, which regulates calcium entry into myocytes. Mutations in these genes cause various phenotypes, including Timothy syndrome, Brugada syndrome, and early repolarization syndrome. The identification of mutations associated with 'calcium-handling diseases' has led to an improved understanding of the role of calcium in cardiac physiology.
Key Points
-
Ca2+ transport in myocardial cells is a key process of cardiac excitability
-
A variety of clinical phenotypes can be caused by genetic mutations in genes controlling calcium handling
-
Mutations can affect both the 'intracellular' (sarcoplasmic reticulum calcium release) and the 'transmembrane' (Ca2+ influx through voltage-dependent L-type calcium channels) components of calcium handling
-
Inherited arrhythmias associated with Ca2+ dysfunction are often severe and life-threatening conditions
-
Risk stratification and therapy for patients with calcium channelopathies can save lives, but several knowledge gaps and uncertainties remain
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Cosmocyte/Ben Smith.

Similar content being viewed by others
References
Bers, D. M. Cardiac excitation–contraction coupling. Nature 415, 198–205 (2002).
Burashnikov, E. et al. Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm 7, 1872–1882 (2010).
Priori, S. G. et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 103, 196–200 (2001).
Lahat, H. et al. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am. J. Hum. Genet. 69, 1378–1384 (2001).
Hasenfuss, G. & Pieske, B. Calcium cycling in congestive heart failure. J. Mol. Cell. Cardiol. 34, 951–969 (2002).
George, C. H. Sarcoplasmic reticulum Ca2+ leak in heart failure: mere observation or functional relevance? Cardiovasc. Res. 77, 302–314 (2008).
Pogwizd, S. M. & Bers, D. M. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc. Med. 14, 61–66 (2004).
Terentyev, D., Viatchenko-Karpinski, S., Valdivia, H. H., Escobar, A. L. & Györke, S. Luminal Ca2+ controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circ. Res. 91, 414–420 (2002).
Bassani J. W., Bassani, R. A. & Bers, D. M. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J. Physiol. 476, 279–293 (1994).
Pieske, B., Maier, L. S., Bers, D. M. & Hassenfuss, G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ. Res. 85, 38–46 (1999).
Cheng, H., Lederer, W. J. & Cannell, M. B. Calcium sparks: elementary events underlying excitation–contraction coupling in heart muscle. Science 262, 740–744 (1993).
Franzini-Armstrong, C., Protasi, F. & Tijskens, P. The assembly of calcium release units in cardiac muscle. Ann. NY Acad. Sci. 1047, 76–85 (2005).
Cannell, M. B., Cheng, H. & Lederer, W. J. The control of calcium release in heart muscle. Science 268, 1045–1049 (1995).
Satoh, H., Blatter, L. A. & Bers, D. M. Effects of [Ca2+]i, SR Ca2+ load, and rest on Ca2+ spark frequency in ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 272, H657–H668 (1997).
Denegri, M. et al. Viral gene transfer rescues arrhythmogenic phenotype and ultrastructural abnormalities in adult calsequestrin-null mice with inherited arrhythmias. Circ. Res. 110, 663–668 (2012).
Ginsburg, K. S. & Bers, D. M. Modulation of excitation–contraction coupling by isoproterenol in cardiomyocytes with controlled SR Ca2+ load and Ca2+ current trigger. J. Physiol. 556, 463–480 (2004).
Hussain, M. & Orchard, C. H. Sarcoplasmic reticulum Ca2+ content, L-type Ca2+ current and the Ca2+ transient in rat myocytes during beta-adrenergic stimulation. J. Physiol. 505, 385–402 (1997).
Grimm, M. & Brown, J. H. Beta-adrenergic receptor signaling in the heart: role of CaMKII. J. Mol. Cell. Cardiol. 48, 322–330 (2010).
Reiken, S. et al. Protein kinase A phosphorylation of the cardiac calcium release channel (ryanodine receptor) in normal and failing hearts. Role of phosphatases and response to isoproterenol. J. Biol. Chem. 278, 444–453 (2003).
Wehrens, X. H., Lehnart, S. E., Reiken, S. R. & Marks, A. R. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ. Res. 94, e61–e70 (2004).
MacDonnell, S. M. et al. Adrenergic regulation of cardiac contractility does not involve phosphorylation of the cardiac ryanodine receptor at serine 2808. Circ. Res. 102, e65–e72 (2008).
Marx, S. O. et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101, 365–376 (2000).
Ai, X., Curran, J. W., Shannon, T. R., Bers, D. M. & Pogwizd S. M. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ. Res. 97, 1314–1322 (2005).
Bers, D. M. Ryanodine receptor S2808 phosphorylation in heart failure: smoking gun or red herring. Circ. Res. 110, 796–799 (2012).
Maier, L. S. & Bers, D. M. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation–contraction coupling in the heart. Cardiovasc. Res. 73, 631–640 (2007).
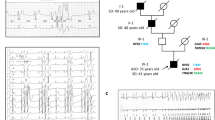
Priori, S. G. et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation 106, 69–74 (2002).
Laitinen, P. J. et al. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation 103, 485–490 (2001).
Bhuiyan, Z. A. et al. Expanding spectrum of human RYR2-related disease: new electrocardiographic, structural, and genetic features. Circulation 116, 1569–1576 (2007).
Tang, Y., Tian, X., Wang, R., Fill, M. & Chen, S. R. Abnormal termination of Ca2+ release is a common defect of RyR2 mutations associated with cardiomyopathies. Circ. Res. 110, 968–977 (2012).
Tiso, N. et al. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2). Hum. Mol. Genet. 10, 189–194 (2001).
Venetucci, L. A., Trafford, A. W., O'Neill, S. C. & Eisner, D. A. The sarcoplasmic reticulum and arrhythmogenic calcium release. Cardiovasc. Res. 77, 285–292 (2008).
Cheng, H., Lederer, M. R., Lederer, W. J. & Cannell, M. B. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am. J. Physiol. 270, C148–C159 (1996).
Lukyanenko, V., Subramanian, S., Györke, I., Wiesner, T. F. & Györke, S. The role of luminal Ca2+ in the generation of Ca2+ waves in rat ventricular myocytes. J. Physiol. 518, 173–186 (1999).
Venetucci, L. A., Trafford, A. W. & Eisner, D. A. Increasing ryanodine receptor open probability alone does not produce arrhythmogenic calcium waves: threshold sarcoplasmic reticulum calcium content is required. Circ. Res. 100, 105–111 (2007).
Trafford, A. W., Sibbring, G. C., Díaz, M. E. & Eisner, D. A. The effects of low concentrations of caffeine on spontaneous Ca release in isolated rat ventricular myocytes. Cell Calcium 28, 269–276 (2000).
Overend, C. L., Eisner, D. A. & O'Neill, S. C. The effect of tetracaine on spontaneous Ca2+ release and sarcoplasmic reticulum calcium content in rat ventricular myocytes. J. Physiol. 502, 471–479 (1997).
Lederer, W. J. & Tsien, R. W. Transient inward current underlying arrhythmogenic effects of cardiotonic steroids in Purkinje fibers. J. Physiol. 263, 73–100 (1976).
Jiang, D. et al. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ. Res. 97, 1173–1181 (2005).
Jiang, D. et al. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR). Proc. Natl Acad. Sci. USA 101, 13062–13067 (2004).
Terentyev, D. et al. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ. Res. 98, 1151–1158 (2006).
Liu, N. et al. Arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia: insights from a RyR2 R4496C knock-in mouse model. Circ. Res. 99, 292–298 (2006).
George, C. H., Jundi, H., Thomas, N. L., Fry, D. L. & Lai, F. A. Ryanodine receptors and ventricular arrhythmias: emerging trends in mutations, mechanisms and therapies. J. Mol. Cell. Cardiol. 42, 34–50 (2007).
Medeiros-Domingo, A. et al. The RYR2-encoded ryanodine receptor/calcium release channel in patients diagnosed previously with either catecholaminergic polymorphic ventricular tachycardia or genotype negative, exercise-induced long QT syndrome: a comprehensive open reading frame mutational analysis. J. Am. Coll. Cardiol. 54, 2065–2074 (2009).
Rousseau, E., Smith, J. S., Henderson, J. S. & Meissner, G. Single channel and 45Ca2+ flux measurements of the cardiac sarcoplasmic reticulum calcium channel. Biophys. J. 50, 1009–1014 (1986).
Sitsapesan, R. & Williams, A. J. Regulation of the gating of the sheep cardiac sarcoplasmic reticulum Ca2+-release channel by luminal Ca2+. J. Membr. Biol. 137, 215–226 (1994).
Stern, M. D. & Cheng, H. Putting out the fire: what terminates calcium-induced calcium release in cardiac muscle? Cell Calcium 35, 591–601 (2004).
IRCCS Fondazione Salvatore Maugeri, Italy and New York University, USA. The gene connection for the heart. Genetic mutations and inherited arrhythmias [online], (2010).
Jones, P. P. et al. Endoplasmic reticulum Ca2+ measurements reveal that the cardiac ryanodine receptor mutations linked to cardiac arrhythmia and sudden death alter the threshold for store-overload-induced Ca2+ release. Biochem. J. 412, 171–178 (2008).
Lehnart, S. E. et al. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation 109, 3208–3214 (2004).
Wehrens, X. H. et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell 113, 829–840 (2003).
George, C. H., Higgs, G. V. & Lai, F. A. Ryanodine receptor mutations associated with stress-induced ventricular tachycardia mediate increased calcium release in stimulated cardiomyocytes. Circ. Res. 93, 531–540 (2003).
Guo, T. et al. Kinetics of FKBP12.6 binding to ryanodine receptors in permeabilized cardiac myocytes and effects on Ca sparks. Circ. Res. 106, 1743–1752 (2010).
Kashimura, T. et al. In the RyR2(R4496C) mouse model of CPVT, β-adrenergic stimulation induces Ca waves by increasing SR Ca content and not by decreasing the threshold for Ca waves. Circ. Res. 107, 1483–1489 (2010).
Yamamoto, T. et al. Identification of target domains of the cardiac ryanodine receptor to correct channel disorder in failing hearts. Circulation 117, 762–772 (2008).
Uchinoumi, H. et al. Catecholaminergic polymorphic ventricular tachycardia is caused by mutation-linked defective conformational regulation of the ryanodine receptor. Circ. Res. 106, 1413–1424 (2010).
Suetomi, T. et al. Mutation-linked defective interdomain interactions within ryanodine receptor cause aberrant Ca2+ release leading to catecholaminergic polymorphic ventricular tachycardia. Circulation 124, 682–694 (2011).
Jiang, D., Chen, W., Wang, R., Zhang, L. & Chen, S. R. Loss of luminal Ca2+ activation in the cardiac ryanodine receptor is associated with ventricular fibrillation and sudden death. Proc. Natl Acad. Sci. USA 104, 18309–18314 (2007).
Györke, S. & Terentyev, D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc. Res. 77, 245–255 (2008).
Wang, S. et al. Crystal structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum. Nat. Struct. Biol. 5, 476–483 (1998).
Mitchell, R. D., Simmerman, H. K. & Jones, L. R. Ca2+ binding effects on protein conformation and protein interactions of canine cardiac calsequestrin. J. Biol. Chem. 263, 1376–1381 (1988).
Györke, I., Hester, N., Jones, L. R. & Györke, S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys. J. 86, 2121–2128 (2004).
Xu, L. & Meissner, G. Regulation of cardiac muscle Ca2+ release channel by sarcoplasmic reticulum lumenal Ca2+. Biophys. J. 75, 2302–2312 (1998).
Knollmann, B. C. et al. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J. Clin. Invest. 116, 2510–2520 (2006).
Qin, J. et al. Luminal Ca2+ regulation of single cardiac ryanodine receptors: insights provided by calsequestrin and its mutants. J. Gen. Physiol. 131, 325–334 (2008).
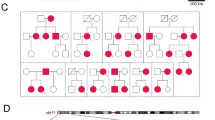
di Barletta, M. R. et al. Clinical phenotype and functional characterization of CASQ2 mutations associated with catecholaminergic polymorphic ventricular tachycardia. Circulation 114, 1012–1019 (2006).
de la Fuente, S., Van Langen, I. M., Postma, A. V., Bikker, H. & Meijer, A. A case of catecholaminergic polymorphic ventricular tachycardia caused by two calsequestrin 2 mutations. Pacing Clin. Electrophysiol. 31, 916–919 (2008).
Rizzi, N. et al. Unexpected structural and functional consequences of the R33Q homozygous mutation in cardiac calsequestrin: a complex arrhythmogenic cascade in a knock-in mouse model. Circ. Res. 103, 298–306 (2008).
Song, L. et al. Calsequestrin 2 (CASQ2) mutations increase expression of calreticulin and ryanodine receptors, causing catecholaminergic polymorphic ventricular tachycardia. J. Clin. Invest. 117, 1814–1823 (2007).
Kalyanasundaram, A., Bal, N. C., Franzini-Armstrong, C., Knollmann, B. C. & Periasamy, M. The calsequestrin mutation CASQ2D307H does not affect protein stability and targeting to the junctional sarcoplasmic reticulum but compromises its dynamic regulation of calcium buffering. J. Biol. Chem. 285, 3076–3083 (2010).
Bal, N. C. et al. The catecholaminergic polymorphic ventricular tachycardia mutation R33Q disrupts the N-terminal structural motif that regulates reversible calsequestrin polymerization. J. Biol. Chem. 285, 17188–17196 (2010).
Valle, G. et al. Catecholaminergic polymorphic ventricular tachycardia-related mutations R33Q and L167H alter calcium sensitivity of human cardiac calsequestrin. Biochem. J. 413, 291–303 (2008).
Houle, T. D., Ram, M. L. & Cala, S. E. Calsequestrin mutant D307H exhibits depressed binding to its protein targets and a depressed response to calcium. Cardiovasc. Res. 64, 227–233 (2004).
Eisner, D. A., Trafford, A. W., Díaz, M. E., Overend, C. L. & O'Neill, S. C. The control of Ca release from the cardiac sarcoplasmic reticulum: regulation versus autoregulation. Cardiovasc. Res. 38, 589–604 (1998).
Liu, N. et al. Calmodulin kinase II inhibition prevents arrhythmias in RyR2(R4496C±) mice with catecholaminergic polymorphic ventricular tachycardia. J. Mol. Cell. Cardiol. 50, 214–222 (2011).
Curran, J., Brown, K. H., Santiago, D. J., Pogwizd, S., Bers, D. M. & Shannon, T. R. Spontaneous Ca waves in ventricular myocytes from failing hearts depend on Ca2+-calmodulin-dependent protein kinase II. J. Mol. Cell. Cardiol. 49, 25–32 (2010).
Leenhardt, A. et al. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation 91, 1512–1519 (1995).
Postma, A. V. et al. Catecholaminergic polymorphic ventricular tachycardia: RYR2 mutations, bradycardia, and follow up of the patients. J. Med. Genet. 42, 863–870 (2005).
Marjamaa, A. et al. Intravenous epinephrine infusion test in diagnosis of catecholaminergic polymorphic ventricular tachycardia. J. Cardiovasc. Electrophysiol. 23, 194–199 (2011).
Landzberg, S., Parker, J. D., Gauthier, D. F. & Colucci, W. S. Effects of intracoronary acetylcholine and atropine on basal and dobutamine-stimulated left ventricular contractility. Circulation 89, 164–168 (1994).
Mohamed, U., Gollob, M. H., Gow, R. M. & Krahn, A. D. Sudden cardiac death despite an implantable cardioverter-defibrillator in a young female with catecholaminergic ventricular tachycardia. Heart Rhythm 3, 1486–1489 (2006).
Odero, A., Bozzani, A., De Ferrari, G. M. & Schwartz, P. J. Left cardiac sympathetic denervation for the prevention of life-threatening arrhythmias: the surgical supraclavicular approach to cervicothoracic sympathectomy. Heart Rhythm 7, 1161–1165 (2010).
Collura, C. A., Johnson, J. N., Moir, C. & Ackerman, M. J. Left cardiac sympathetic denervation for the treatment of long QT syndrome and catecholaminergic polymorphic ventricular tachycardia using video-assisted thoracic surgery. Heart Rhythm 6, 752–759 (2009).
Wilde, A. A. et al. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N. Engl. J. Med. 358, 2024–2029 (2008).
Watanabe, H. et al. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat. Med. 15, 380–383 (2009).
van der Werf, C. et al. Flecainide therapy reduces exercise-induced ventricular arrhythmias in patients with catecholaminergic polymorphic ventricular tachycardia. J. Am. Coll. Cardiol. 57, 2244–2254 (2011).
Hilliard, F. A. et al. Flecainide inhibits arrhythmogenic Ca2+ waves by open state block of ryanodine receptor Ca2+ release channels and reduction of Ca2+ spark mass. J. Mol. Cell. Cardiol. 48, 293–301 (2010).
Conard, G. J. & Ober, R. E. Metabolism of flecainide. Am. J. Cardiol. 53, 41B–51B (1984).
Liu, H., Atkins, J. & Kass, R. S. Common molecular determinants of flecainide and lidocaine block of heart Na+ channels: evidence from experiments with neutral and quaternary flecainide analogs. J. Gen. Physiol. 121, 199–214 (2003).
Liu, N. et al. Short communication: flecainide exerts an antiarrhythmic effect in a mouse model of catecholaminergic polymorphic ventricular tachycardia by increasing the threshold for triggered activity. Circ. Res. 109, 291–295 (2011).
Lehnart, S. E. et al. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation 109, 3208–3214 (2004).
Loughrey, C. M. et al. K201 modulates excitation–contraction coupling and spontaneous Ca2+ release in normal adult rabbit ventricular cardiomyocytes. Cardiovasc. Res. 76, 236–246 (2007).
Sedej, S. et al. Na+-dependent SR Ca2+ overload induces arrhythmogenic events in mouse cardiomyocytes with a human CPVT mutation. Cardiovasc. Res. 87, 50–59 (2010).
Napolitano, C. & Antzelevitch, C. Phenotypical manifestations of mutations in the genes encoding subunits of the cardiac voltage-dependent L-type calcium channel. Circ. Res. 108, 607–618 (2011).
Yuan, W., Ginsburg, K. S. & Bers, D. M. Comparison of sarcolemmal calcium channel current in rabbit and rat ventricular myocytes. J. Physiol. 493, 733–746 (1996).
Bers, D. M. & Perez-Reyes, E. Ca channels in cardiac myocytes: structure and function in Ca influx and intracellular Ca release. Cardiovasc. Res. 42, 339–360 (1999).
Zhang, Z. et al. Functional roles of Cav1.3(α1D) calcium channels in sinoatrial nodes: insights gained from gene-targeted null mutant mice. Circ. Res. 90, 981–987 (2002).
Mikami, A. et al. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature 340, 230–233 (1989).
Hulme, J. T., Yarov-Yarovoy, V., Lin, T. W., Scheuer, T. & Catterall, W. A. Autoinhibitory control of the CaV1.2 channel by its proteolytically processed distal C-terminal domain. J. Physiol. 576, 87–102 (2006).
Fu, Y. et al. Deletion of the distal C terminus of CaV1.2 channels leads to loss of beta-adrenergic regulation and heart failure in vivo. J. Biol. Chem. 286, 12617–12626 (2011).
Catterall, W. A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 3, a003947 (2011).
Davies, A. et al. The α2δ subunits of voltage-gated calcium channels form GPI-anchored proteins, a post-translational modification essential for function. Proc. Natl Acad. Sci. USA 107, 1654–1659 (2010).
Fuller-Bicer, G. A. et al. Targeted disruption of the voltage-dependent calcium channel α2/δ-1-subunit. Am. J. Physiol. Heart Circ. Physiol. 297, H117–H124 (2009).
Colecraft, H. M. et al. Novel functional properties of Ca2+ channel beta subunits revealed by their expression in adult rat heart cells. J. Physiol. 541, 435–452 (2002).
Buraei, Z. & Yang, J. The β subunit of voltage-gated Ca2+ channels. Physiol. Rev. 90, 1461–1506 (2010).
Ruan, Y., Liu, N. & Priori, S. G. Sodium channel mutations and arrhythmias. Nat. Rev. Cardiol. 6, 337–348 (2009).
Bezzina, C. et al. A single Na+ channel mutation causing both long-QT and Brugada syndromes. Circ. Res. 85, 1206–1213 (1999).
Antzelevitch, C. et al. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation 115, 442–449 (2007).
Cordeiro, J. M. et al. Accelerated inactivation of the L-type calcium current due to a mutation in CACNB2b underlies Brugada syndrome. J. Mol. Cell. Cardiol. 46, 695–703 (2009).
Templin, C. et al. Identification of a novel loss-of-function calcium channel gene mutation in short QT syndrome (SQTS6). Eur. Heart J. 32, 1077–1088 (2011).
Gollob, M. H. et al. Recommendations for the use of genetic testing in the clinical evaluation of inherited cardiac arrhythmias associated with sudden cardiac death: Canadian Cardiovascular Society/Canadian Heart Rhythm Society joint position paper. Can. J. Cardiol. 27, 232–245 (2011).
Brugada, P. & Brugada, J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J. Am. Coll. Cardiol. 20, 1391–1396 (1992).
Antzelevitch, C. et al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 111, 659–670 (2005).
Wilde, A. A. et al. The pathophysiological mechanism underlying Brugada syndrome: depolarization versus repolarization. J. Mol. Cell. Cardiol. 49, 543–553 (2010).
Yan, G. X. & Antzelevitch, C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation 100, 1660–1666 (1999).
Meregalli, P. G., Wilde, A. A. & Tan, H. L. Pathophysiological mechanisms of Brugada syndrome: depolarization disorder, repolarization disorder, or more? Cardiovasc. Res. 67, 367–378 (2005).
Priori, S. G. et al. Natural history of Brugada syndrome: insights for risk stratification and management. Circulation 105, 1342–1347 (2002).
Priori, S. G. et al. Risk stratification in Brugada syndrome: results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) registry. J. Am. Coll. Cardiol. 59, 37–45 (2012).
Probst, V. et al. Long-term prognosis of patients diagnosed with Brugada syndrome: results from the FINGER Brugada Syndrome Registry. Circulation 121, 635–643 (2010).
Gehi, A. K., Duong, T. D., Metz, L. D., Gomes, J. A. & Mehta, D. Risk stratification of individuals with the Brugada electrocardiogram: a meta-analysis. J. Cardiovasc. Electrophysiol. 17, 577–583 (2006).
Ohgo, T. et al. Acute and chronic management in patients with Brugada syndrome associated with electrical storm of ventricular fibrillation. Heart Rhythm 4, 695–700 (2007).
Belthassen, B., Glick, A. & Viskin, S. Efficacy of quinidine in high-risk patients with Brugada syndrome. Circulation 110, 1731–1737 (2004).
Antzelevitch, C. & Fish, J. M. Therapy for the Brugada syndrome. Handb. Exp. Pharmacol. 171, 305–330 (2006).
Bjerregaard, P., Nallapaneni, H. & Gussak, I. Short QT interval in clinical practice. J. Electrocardiol. 43, 390–395 (2010).
Viskin, S. The QT interval: too long, too short or just right. Heart Rhythm 6, 711–715 (2009).
Gollob, M. H., Redpath, C. J. & Roberts, J. D. The short QT syndrome: proposed diagnostic criteria. J. Am. Coll. Cardiol. 57, 802–812 (2011).
Brugada, R. et al. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation 109, 30–35 (2004).
Bellocq, C. et al. Mutation in the KCNQ1 gene leading to the short QT-interval syndrome. Circulation 109, 2394–2397 (2004).
Priori, S. G. et al. A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ. Res. 96, 800–807 (2005).
Patel, C., Yan, G. X. & Antzelevitch, C. Short QT syndrome: from bench to bedside. Circ. Arrhythm. Electrophysiol. 3, 401–408 (2010).
Gaita, F. et al. Short QT syndrome: pharmacologic treatment. J. Am. Coll. Cardiol. 43, 1494–1499 (2004).
Giustetto, C. et al. Long term follow-up of patients with short QT syndrome. J. Am. Coll. Cardiol. 58, 587–595 (2011).
Iost, N., Viràg, L., Varrò, A. & Papp, J. G. Comparison of the effect of class IA antiarrhythmic drugs on transmembrane potassium currents in rabbit ventricular myocytes. J. Cardiovasc. Pharmacol. Ther. 8, 31–41 (2003).
Boineau, J. P. The early repolarization variant—normal or a marker of heart disease in certain subjects. J. Electrocardiol. 40, 3.e11–3.e16 (2007).
Haïssaguerre, M. et al. Sudden cardiac arrest associated with early repolarization. N. Engl. J. Med. 358, 2016–2023 (2008).
Rosso, R. et al. J-point elevation in survivors of primary ventricular fibrillation and matched control subjects: incidence and clinical significance. J. Am Coll. Cardiol. 52, 1231–1238 (2008).
Zipes, D. P. et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J. Am. Coll. Cardiol. 48, e247–e346 (2006).
Rosso, R., Adler, A., Halkin, A. & Viskin, S. Risk of sudden death among young individuals with J waves and early repolarization: putting the evidence into perspective. Heart Rhythm 8, 923–929 (2011).
Tikkanen, J. T. et al. Early repolarization: electrocardiographic phenotypes associated with favorable long-term outcome. Circulation 123, 2666–2673 (2011).
Rosso, R. et al. Distinguishing “benign” from “malignant early repolarization”: the value of the ST-segment morphology. Heart Rhythm 9, 225–229 (2011).
Antzelevitch, C. & Yan, G. X. J-wave syndromes: from cell to bedside. J. Electrocardiol. 44, 656–661 (2011).
Haïssaguerre, M. et al. Characteristics of recurrent ventricular fibrillation associated with inferolateral early repolarization role of drug therapy. J. Am. Coll. Cardiol. 53, 612–619 (2009).
Letsas, K. P. et al. Prevalence of early repolarization pattern in inferolateral leads in patients with Brugada syndrome. Heart Rhythm 5, 1685–1689 (2008).
Watanabe, H. et al. High prevalence of early repolarization in short QT syndrome. Heart Rhythm 7, 647–652 (2010).
Splawski, I. et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell 119, 19–31 (2004).
Gillis, J. et al. Long QT, syndactyly, joint contractures, stroke and novel CACNA1C mutation: expanding the spectrum of Timothy syndrome. Am. J. Med. Genet. A. http://dx.doi.org/10.1002/ajmg.a.34355.
Splawski, I. et al. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc. Natl Acad. Sci. USA 102, 8089–8096 (2005).
Etheridge, S. P. et al. Somatic mosaicism contributes to phenotypic variation in Timothy syndrome. Am. Med. Genet. A. 155, 2578–2583 (2011).
Barrett, C. F. & Tsien, R. W. The Timothy syndrome mutation differentially affects voltage- and calcium-dependent inactivation of CaV1.2 L-type calcium channels. Proc. Natl Acad. Sci. USA 105, 2157–2162 (2008).
Yazawa, M. et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 471, 230–234 (2011).
Pas¸ca, S. P. et al. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat. Med. 17, 1657–1662 (2011).
Bader, P. L. et al. Mouse model of Timothy syndrome recapitulates triad of autistic traits. Proc. Natl Acad. Sci. USA 108, 15432–15437 (2011).
Jacobs, A., Knight, B. P., McDonald, K. T. & Burke, M. C. Verapamil decreases ventricular tachyarrhythmias in a patient with Timothy syndrome (LQT8). Heart Rhythm 3, 967–970 (2006).
Sung, R. J. et al. Beta-adrenergic modulation of arrhythmogenesis and identification of targeted sites of antiarrhythmic therapy in Timothy (LQT8) syndrome: a theoretical study. Am. J. Physiol. Heart Circ. Physiol. 298, H33–H44 (2010).
Sicouri, S. et al. Cellular basis for the electrocardiographic and arrhythmic manifestations of Timothy syndrome: effects of ranolazine. Heart Rhythm 4, 638–647 (2007).
Johns Hopkins University and US National Library of Medicine. Online Mendelian Inheritance in Man (OMIM) [online], (2012).
Acknowledgements
Part of the data presented here has been produced thanks to the contribution of the following research grants: Telethon grants GGP04066, GGP06007, and GGP11141 (to S. G. Priori), Italian University Research and Technology RFMAU207641137D (to S. G. Priori), Fondation Leducq Award to the Alliance for Calmodulin Kinase Signaling in Heart Disease (08CVD01) (to S. G. Priori), Fondation Veronesi “Modelli cellulari e terapia sperimentale dei difetti cardiaci associati a patologie aritmogene ereditarie” (to S. G. Priori). CARIPLO pr.2008.2275 (to S. G. Priori), and British Heart Foundation Intermediate Clinical Research Fellowship FS10/63/28,374 (to L. Venetucci and S. G. Priori).
Author information
Authors and Affiliations
Contributions
All authors contributed to the discussion of content for this Review. In addition, L. Venetucci researched data for and wrote the article, M. Denegri and C. Napolitano reviewed/edited the manuscript before submission, and S. G. Priori wrote the article and reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Venetucci, L., Denegri, M., Napolitano, C. et al. Inherited calcium channelopathies in the pathophysiology of arrhythmias. Nat Rev Cardiol 9, 561–575 (2012). https://doi.org/10.1038/nrcardio.2012.93
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2012.93
This article is cited by
-
Network-Based Data Analysis Reveals Ion Channel-Related Gene Features in COVID-19: A Bioinformatic Approach
Biochemical Genetics (2023)
-
Metabolically driven maturation of human-induced-pluripotent-stem-cell-derived cardiac microtissues on microfluidic chips
Nature Biomedical Engineering (2022)
-
A SPRY1 domain cardiac ryanodine receptor variant associated with short-coupled torsade de pointes
Scientific Reports (2021)
-
Bone mineral density and risk of cardiovascular disease in men and women: the HUNT study
European Journal of Epidemiology (2021)
-
WWP2 and PPP1R3A are abnormally regulated in arrhythmia-induced cardiac damage
3 Biotech (2021)