Key Points
-
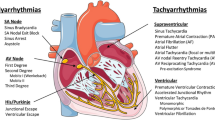
Ventricular arrhythmias are the most common cause of sudden death, and individual risk prediction is a major medical challenge
-
The His–Purkinje system constitutes a tiny fraction of the ventricular mass, but is specialized for rapid synchronous activation of both ventricles
-
The pathogenic role of the Purkinje system is disproportionately high; discrete excitations act as main triggers of ventricular fibrillation in individuals with structurally normal hearts and in patients with diseased hearts
-
Purkinje cells are known to have a unique electrophysiology involving complex intracellular Ca2+ cycling
-
Further characterization of arrhythmia mechanisms is needed to enable the development of targeted therapy
Abstract
Ventricular arrhythmias are a major cause of sudden death, which accounts for approximately half of cardiac mortality. The His–Purkinje system is composed of specialized cells responsible for the synchronous activation of the ventricles. However, experimental studies show that the Purkinje system can be arrhythmogenic during electrolyte imbalance, after exposure to various drugs, and in myocardial ischaemia, during which Purkinje cells can survive in anaerobic conditions. Purkinje cells can generate both automatic and triggered focal rhythms, and their network configuration can accommodate re-entrant circuits. In humans, a variety of monomorphic ventricular tachycardias can be sustained within the architecture of the Purkinje branches. Furthermore, discrete Purkinje sources can serve as critical triggers of ventricular fibrillation in a wide spectrum of patients with structural heart disease or with an apparently normal heart. In drug-resistant cases of monomorphic and polymorphic Purkinje-related ventricular tachycardias, catheter ablation is a very effective treatment. The specific transcriptional signatures and functional properties of Purkinje cells, including their intracellular calcium dynamics, underlie their extreme arrhythmogenicity. However, the identification of vulnerable individuals remains challenging, and the molecular mechanisms of Purkinje-related arrhythmias have to be characterized further to enable the development of interventions to prevent lethal cardiac arrhythmias.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
John, R. M. et al. Ventricular arrhythmias and sudden cardiac death. Lancet 380, 1520–1529 (2012).
Marsman, R. F., Tan, H. L. & Bezzina, C. R. Genetics of sudden cardiac death caused by ventricular arrhythmias. Nat. Rev. Cardiol. 11, 96–111 (2014).
Tung, P. & Albert, C. M. Causes and prevention of sudden cardiac death in the elderly. Nat. Rev. Cardiol. 10, 135–142 (2013).
Silverman, M. E., Grove, D., Upshaw, C. B. Jr. Why does the heart beat? The discovery of the electrical system of the heart. Circulation 113, 2775–2781 (2006).
Scheinman, M. M. Role of the His−Purkinje system in the genesis of cardiac arrhythmia. Heart Rhythm 6, 1050–1058 (2009).
Boyden, P. A., Hirose, M. & Dun, W. Cardiac Purkinje cells. Heart Rhythm 7, 127–135 (2010).
Ono, N. et al. Morphological varieties of the Purkinje fiber network in mammalian hearts, as revealed by light and electron microscopy. Arch. Histol. Cytol. 72, 139–149 (2009).
Demoulin, J. C. & Kulbertus, H. E. Histopathological examination of concept of left hemiblock. Br. Heart J. 34, 807–814 (1972).
Christoffels, V. M. & Moorman, A. F. Development of the cardiac conduction system: why are some regions of the heart more arrhythmogenic than others? Circ. Arrhythm. Electrophysiol. 2, 195–207 (2009).
Dobrzynski, H. et al. Structure, function and clinical relevance of the cardiac conduction system, including the atrioventricular ring and outflow tract tissues. Pharmacol. Ther. 139, 260–288 (2013).
Sommer, J. R. & Johnson, E. A. A comparative study of Purkinje fibers and ventricular fibers. J. Cell Biol. 36, 497–526 (1968).
Eisenberg, B. R. & Cohen, I. S. The ultrastructure of the cardiac Purkinje strand in the dog: a morphometric analysis. Proc. R. Soc. Lond. B Biol. Sci. 217, 191–213 (1983).
Sommer, J. R. & Johnson, E. A. Purkinje fibers of the heart examined with the peroxidase reaction. J. Cell Biol. 37, 570–574 (1968).
Desplantez, T., Dupont, E., Severs, N. J. & Weingart, R. Gap junction channels and cardiac impulse propagation. J. Membr. Biol. 218, 13–28 (2007).
Gourdie, R. G. et al. The spatial distribution and relative abundance of gap-junctional connexin40 and connexin43 correlate to functional properties of components of the cardiac atrioventricular conduction system. J. Cell Sci. 105, 985–991 (1993).
Gaborit, N. et al. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J. Physiol. 582, 675–693 (2007).
Gintant, G. A., Datyner, N. B. & Cohen, I. S. Slow inactivation of a tetrodotoxin-sensitive current in canine cardiac Purkinje fibers. Biophys. J. 45, 509–512 (1984).
Stuyvers, B. D. et al. Ca2+ sparks and waves in canine Purkinje cells: a triple layered system of Ca2+ activation. Circ. Res. 97, 35–43 (2005).
Dun, W. & Boyden, P. A. The Purkinje cell; 2008 style. J. Mol. Cell Cardiol. 45, 617–624 (2008).
Ter Keurs, H. E. & Boyden, P. A. Calcium and arrhythmogenesis. Physiol. Rev. 87, 457–506 (2007).
Segers, M. Le battement auto-entretenu du cœur [French]. Arch. Int. Pharmacodyn. 75, 144–156 (1947).
Cranefield, P. F. Action potentials, afterpotentials, and arrhythmias. Circ. Res. 41, 415–423 (1977).
Song, Z., Ko, C. Y., Nivala, M., Weiss, J. N. & Qu, Z. Calcium-voltage coupling in the genesis of early and delayed afterdepolarizations in cardiac myocytes. Biophys. J. 108, 1908–1921 (2015).
Hirose, M., Stuyvers, B. D., Dun, W., Ter Keurs, H. E. D. J. & Boyden, P. A. Function of Ca2+ release channels in purkinje cells that survive in the infarcted canine heart: a mechanism for triggered Purkinje ectopy. Circ. Arrhythm. Electrophysiol. 1, 387–395 (2008).
De Lange, E., Xie, Y. & Qu, Z. Synchronization of early afterdepolarizations and arrhythmogenesis in heterogeneous cardiac tissue models. Biophys. J. 103, 365–373 (2012).
Nivala, M., Ko, C. Y., Nivala, M., Weiss, J. N. & Qu, Z. Criticality in intracellular calcium signaling in cardiac myocytes. Biophys. J. 102, 2433–2442 (2012).
Maguy, A. et al. Ion channel subunit expression changes in cardiac Purkinje fibers: a potential role in conduction abnormalities associated with congestive heart failure. Circ. Res. 104, 1113–1122 (2009).
Deo, M., Boyle, P. M., Kim, A. M. & Vigmond, E. J. Arrhythmogenesis by single ectopic beats originating in the Purkinje system. Am. J. Physiol. Heart Circ. Physiol. 299, H1002–H1011 (2010).
Tranum-Jensen, J., Wilde, A. A., Vermeulen, J. T. & Janse, M. J. Morphology of electrophysiologically identified junctions between Purkinje fibers and ventricular muscle in rabbit and pig hearts. Circ. Res. 69, 429–437 (1991).
Martinez-Palomo, A., Alanis, J. & Benitex, D. Transitional cardiac cells of the conductive system of the dog heart: distinguishing morphological and electrophysiological features. J. Cell Biol. 47, 1–17 (1970).
Joyner, R. W. & Overholt, E. D. Effects of octanol on canine subendocardial Purkinje-to-ventricular transmission. Am. J. Physiol. 249, H1228–H1231 (1985).
Boyle, P. M., Vigmond, E. J. An intuitive safety factor for cardiac propagation. Biophys. J. 98, L57–L59 (2010).
Gilmour, R. F. Jr & Moïse, N. S. Triggered activity as a mechanism for inherited ventricular arrhythmias in German shepherd Dogs. J. Am. Coll. Cardiol. 27, 1526–1533 (1996).
Myerburg, R. J., Nilsson, K. & Gelband, H. Physiology of canine intraventricular conduction and endocardial excitation. Circ. Res. 30, 217–243 (1972).
Myerburg, R. J., Stewart, J. W. & Hoffman, B. F. Electrophysiological properties of the canine peripheral A-V conducting system. Circ. Res. 26, 361–378 (1970).
Overholt, E. D., Joyner, R. W., Veenstra, R. D., Rawling, D. & Wiedmann, R. Unidirectional block between Purkinje and ventricular layers of papillary muscles. Am. J. Physiol. 247, H584–H595 (1984).
Morley, G. E. et al. Reduced intercellular coupling leads to paradoxical propagation across the Purkinje−ventricular junction and aberrant myocardial activation. Proc. Natl Acad. Sci. USA 102, 4126–4129 (2005).
Rohr, S., Kucera, J. P., Fast, V. G. & Kléber, A. G. Paradoxical improvement of impulse conduction in cardiac tissue by partial cellular uncoupling. Science 275, 841–844 (1997).
Tusscher, K. H. & Panfilov, A. V. Modelling of the ventricular conduction system. Prog. Biophys. Mol. Biol. 96, 152–170 (2008).
Berenfeld, O. & Jalife, J. Purkinje-muscle reentry as a mechanism of polymorphic ventricular arrhythmias in a 3-dimensional model of the ventricles. Circ. Res. 82, 1063–1077 (1998).
Kusa, S. et al. Bundle branch reentrant ventricular tachycardia with wide and narrow QRS morphology. Circ. Arrhythm. Electrophysiol. 6, e87–e91 (2013).
Mizusawa, Y. et al. Characteristics of bundle branch reentrant ventricular tachycardia with a right bundle branch block configuration: feasibility of atrial pacing. Europace 11, 1208–1213 (2009).
Schmidt, B. et al. Left bundle branch−Purkinje system in patients with bundle branch reentrant tachycardia: lessons from catheter ablation and electroanatomic mapping. Heart Rhythm 6, 51–58 (2009).
Nogami, A. Purkinje-related arrhythmias part I: monomorphic ventricular tachycardias. Pacing Clin. Electrophysiol. 34, 624–650 (2011).
Belhassen, B., Rotmensch, H. H. & Laniado, S. Response of recurrent sustained ventricular tachycardia to verapamil. Br. Heart J. 46, 679–682 (1981).
Yeh, S. J., Wen, M. S., Wang, C. C., Lin, F. C. & Wu, D. Adenosine-sensitive ventricular tachycardia from the anterobasal left ventricle. J. Am. Coll. Cardiol. 30, 1339–1345 (1997).
Ip, J. E. et al. Unifying mechanism of sustained idiopathic atrial and ventricular annular tachycardia. Circ. Arrhythm. Electrophysiol. 7, 436–444 (2014).
Tsuchiya, T. et al. Significance of late diastolic potential preceding Purkinje potential in verapamil-sensitive idiopathic left ventricular tachycardia. Circulation 99, 2408–2413 (1999).
Morishima, I., Norgami, A., Tsuboi, H. & Sone, T. Verapamil-sensitive left anterior fascicular ventricular tachycardia associated with a healed myocardial infarction: changes in the delayed Purkinje potential during sinus rhythm. J. Interv. Card. Electrophysiol. 22, 233–237 (2008).
Tsuchiya, T., Okumura, K., Honda, T., Iwasa, A. & Ashikaga, K. Effects of verapamil and lidocaine on two components of the re-entry circuit of verapamil-senstitive idiopathic left ventricular tachycardia. J. Am. Coll. Cardiol. 37, 1415–1421 (2001).
Friedman, P. L., Stewart, J. R., Fenoglio, J. J. Jr & Wit, A. L. Survival of subendocardial Purkinje fibers after extensive myocardial infarction in dogs. Circ. Res. 33, 597–611 (1973).
Lazzara, R., el-Sherif, N. & Scherlag, B. J. Electrophysiological properties of canine Purkinje cells in one-day-old myocardial infarction. Circ. Res. 33, 722–734 (1973).
Scherlag, B. J., El-Sherif, N., Hope, R. & Lazzara, R. Characterization and localization of ventricular arrhythmias resulting from myocardial ischemia and infarction. Circ. Res. 35, 372–383 (1974).
Janse, M. J. & Kléber, A. G. Electrophysiological changes and ventricular arrhythmias in the early phase of regional myocardial ischemia. Circ. Res. 49, 1069–1081 (1981).
El-Sherif, N., Mehra, R., Gough, W. B. & Zeiler, R. H. Ventricular activation patterns of spontaneous and induced ventricular rhythms in canine one-day-old myocardial infarction. Evidence for focal and reentrant mechanisms. Circ. Res. 51, 152–166 (1982).
Gilmour, R. F. Jr, Evans, J. J. & Zipes, D. P. Purkinje-muscle coupling and endocardial response to hyperkalemia, hypoxia, and acidosis. Am. J. Physiol. 247, H303–H311 (1984).
Janse, M. J., Kleber, A. G., Capucci, A., Coronel, R. & Wilms-Schopman, F. Electrophysiological basis for arrhythmias caused by acute ischemia: role of the subendocardium. J. Mol. Cell Cardiol. 18, 339–355 (1986).
Dean, J. W. & Lab, M. J. Arrhythmia in heart failure: role of mechanically induced changes in electrophysiology. Lancet 1, 1309–1311 (1989).
Han, W., Chartier, D., Li, D. & Nattel, S. Ionic remodeling of cardiac Purkinje cells by congestive heart failure. Circulation 104, 2095–2100 (2001).
Nattel, S., Maguy, A., Le Bouter, S. & Yeh, Y. H. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol. Rev. 87, 425–456 (2007).
Cerrone, M. et al. Arrhythmogenic mechanisms in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circ. Res. 101, 1039–1048 (2007).
Kang, G. et al. Purkinje cells from RyR2 mutant mice are highly arrhythmogenic but responsive to targeted therapy. Circ. Res. 107, 512–519 (2010).
El-Sherif, N., Zeiler, R. H., Craelius, W., Gough, W. B. & Henkin, R. QTU prolongation and polymorphic ventricular tachyarrhythmias due to bradycardia dependent early afterdepolarizations. Circul. Res. 63, 286–305 (1988).
El-Sherif, N., Caref, E. B., Yin, H. & Restivo, M. The electrophysiological mechanism of ventricular tachyarrhythmias in the long QT syndrome: tridimensional mapping of activation and recovery patterns. Circul. Res. 79, 474–492 (1996).
Asano, Y., Davidenko, J. M., Baxter, W. T., Gary, R. A. & Jalife, J. Optical mapping of drug-induced polymorphic arrhythmias and torsade de pointes in the isolated rabbit heart. J. Am. Coll. Cardiol. 29, 831–842 (1997).
Choi, B. R., Burton, F. & Salama, G. Cytosolic Ca2+ triggers early afterdepolarization and torsade de pointes in rabbit hearts with type 2 long QT syndrome. J. Physiol. 543, 615–631 (2002).
Maruyama, M. et al. Diastolic intracellular calcium-membrane voltage coupling gain and postshock arrhythmias: role of Purkinje fibers and triggered activity. Circ. Res. 106, 399–408 (2010).
Haïssaguerre, M. et al. Role of Purkinje conducting system in triggering idiopathic sudden cardiac death. Lancet 359, 677–678 (2002).
Haïssaguerre, M. et al. Mapping and ablation of idiopathic ventricular fibrillation. Circulation 106, 962–967 (2002).
Nogami, A., Sugiyasu, A., Kubota, S. & Kato, K. Mapping and ablation of idiopathic ventricular fibrillation from the Purkinje system. Heart Rhythm 2, 646–649 (2005).
Szumowski, L. et al. Mapping and ablation of polymorphic ventricular tachycardia after myocardial infarction. J. Am. Coll. Cardiol. 4, 1700–1706 (2004).
Marrouche, N. F. et al. Mode of initiation and ablation of ventricular fibrillation storms in patients with ischemic cardiomyopathy. J. Am. Coll. Cardiol. 43, 1715–1720 (2004).
Peichl, P. et al. Catheter ablation of arrhythmic storm triggered by monomorphic ectopic beats in patients with coronary artery disease. J. Interv. Card. Electrophysiol. 27, 51–59 (2010).
Kozeluhova, M. et al. Catheter ablation of electrical storm in patients with structural heart disease. Europace 13, 109–113 (2011).
Bode, K. et al. Ablation of polymorphic ventricular tachycardias in patients with structural heart disease. Pacing Clin. Electrophysiol. 31, 1585–1591 (2008).
Deneke, T. et al. Catheter ablation of electrical storm in a collaborative hospital network. Am. J. Cardiol. 108, 233–239 (2011).
Enjoji, Y. et al. Catheter ablation of fatal ventricular tachyarrhythmias storm in acute coronary syndrome — role of Purkinje fiber network. J. Interv. Card. Electrophysiol. 26, 207–215 (2009).
Nogami, A., Kubota, S., Adachi, M. & Igawa, O. Electrophysiologic and histopathologic findings of the ablation sites for ventricular fibrillation in a patient with ischemic cardiomyopathy. J. Interv. Card. Electrophysiol. 24, 133–137 (2009).
Koz´luk, E. et al. Efficacy of catheter ablation in patients with an electrical storm. Kardiol. Pol. 69, 665–670 (2011).
Sinha, A. M. et al. Role of left ventricular scar and Purkinje-like potentials during mapping and ablation of ventricular fibrillation in dilated cardiomyopathy. Pacing Clin. Electrophysiol. 32, 286–290 (2009).
Santoro, F. et al. Ventricular fibrillation triggered by PVCs from papillary muscles: clinical features and ablation. J. Cardiovasc. Electrophysiol. 25, 1158–1164 (2014).
Van Herendael, H. et al. Catheter ablation of ventricular fibrillation: importance of left ventricular outflow tract and papillary muscle triggers. Heart Rhythm 11, 566–573 (2014).
Yokoshiki, H., Mitsuyama, H., Watanabe, M., Sakurai, M. & Tsutsui, H. Pleomorphic ventricular tachycardia originating from Purkinje fiber network of left anterior fascicle. J. Electrocardiol. 43, 452–458 (2010).
Li, Y. G., Grönefeld, G., Israel, C. & Hohnloser, S. H. Catheter ablation of frequently recurring ventricular fibrillation in a patient after aortic valve repair. J. Cardiovasc. Electrophysiol. 15, 90–93 (2004).
Mlcochova, H. et al. Catheter ablation of ventricular fibrillation storm in patients with infiltrative amyloidosis of the heart. J. Cardiovasc. Electrophysiol. 17, 426–430 (2006).
Nakabayashi, K., Sugiura, R. & Oka, T. Catheter ablation targeting Purkinje potentials controlled ventricular fibrillation in a patient with a malignant lymphoma occurring in the ventricular septum. BMJ Case Rep. http://dx.doi.org/10.1136/bcr-2014-209026.
Leenhardt, A. et al. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation 91, 1512–1519 (1995).
Priori, S. G. et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 103, 196–200 (2001).
Kaneshiro, T. et al. Successful catheter ablation of bidirectional ventricular premature contractions triggering ventricular fibrillation in catecholaminergic polymorphic ventricular tachycardia with RyR2 mutation. Circ. Arrhythm. Electrophysiol. 5, e14–e17 (2012).
Roche, M., Renauleaud, C., Ballet, V., Doubovetzky, M. & Guillon, J. M. The isolated rabbit heart and Purkinje fibers as models for identifying proarrhythmic liability. J. Pharmacol. Toxicol. Methods 61, 238–250 (2010).
Nogami, A. Purkinje-related arrhythmias part II: polymorphic ventricular tachycardia and ventricular fibrillation. Pacing Clin. Electrophysiol. 34, 1034–1049 (2011).
Srivathsan, K., Gami, A. S., Ackerman, M. J. & Asirvatham, S. J. Treatment of ventricular fibrillation in a patient with prior diagnosis of long QT syndrome: importance of precise electrophysiologic diagnosis to successfully ablate the trigger. Heart Rhythm 4, 1090–1093 (2007).
Tan, V. H., Yap, J., Hsu, L. F. & Liew, R. Catheter ablation of ventricular fibrillation triggers and electrical storm. Europace 14, 1687–1695 (2012).
Sanchez-Munoz, J. J. et al. Ablation of premature ventricular complexes Triggering Ventricular Fibrillation in a patient with long QT syndrome. Indian Pacing Electrophysiol. J. 11, 81–83 (2011).
Haissaguerre, M. et al. Mapping and ablation of ventricular fibrillation associated with long-QT and Brugada Syndromes. Circulation 108, 925–928 (2003).
Knecht, S. et al. Long-term follow-up of idiopathic ventricular fibrillation ablation: a multicenter study. J. Am. Coll. Cardiol. 54, 522–528 (2009).
Yagishita, A., Yamauchi, Y., Obayashi, T. & Hirao, K. Idiopathic ventricular fibrillation associated with early repolarization which was unmasked by a sodium channel blocker after catheter ablation of atrial fibrillation. J. Interv. Card. Electrophysiol. 41, 145–146 (2014).
Pasquié, J. L. et al. Fever as a precipitant of idiopathic ventricular fibrillation in patients with normal hearts. J. Cardiovasc. Electrophysiol. 15, 1271–1276 (2004).
Kimura, T. et al. Ventricular fibrillation associated with J-wave manifestation following pericarditis after catheter ablation for paroxysmal atrial fibrillation. Can. J. Cardiol. 29, 1330.e1–1330.e3 (2013).
Huang, J. et al. The importance of Purkinje activation in long duration ventricular fibrillation. J. Am. Heart Assoc. 3, e000495 (2014).
Lin, C. et al. Endocardial focal activation originating from Purkinje fibers plays a role in the maintenance of long duration ventricular fibrillation. Croat. Med. J. 55, 121–127 (2014).
Sivagangabalan, G. et al. Regional ion channel gene expression heterogeneity and ventricular fibrillation dynamics in human hearts. PLoS ONE 9, e82179 (2014).
Li, L. et al. Different types of long-duration ventricular fibrillation: can they be identified by electrocardiography. J. Electrocardiol. 45, 658–659 (2012).
Boyle, P. M., Massé, S., Nanthakumar, K. & Vigmond, E. J. Transmural IK(ATP) heterogeneity as a determinant of activation rate gradient during early ventricular fibrillation: mechanistic insights from rabbit ventricular models. Heart Rhythm 10, 1710–1717 (2013).
Damiano, R. J. Jr et al. The effect of chemical ablation of the endocardium on ventricular fibrillation threshold. Circulation 74, 645–652 (1986).
Dosdall, D. J. et al. Chemical ablation of the Purkinje system causes early termination and activation rate slowing of long-duration ventricular fibrillation in dogs. Am. J. Physiol. Heart Circ. Physiol. 295, H883–H889 (2008).
Pak, H. N. et al. Both Purkinje cells and left ventricular posteroseptal reentry contribute to the maintenance of ventricular fibrillation in open-chest dogs and swine: effects of catheter ablation and the ventricular cut-and-sew operation. Circ. J. 72, 1185–1192 (2008).
Zamiri, N. et al. Dantrolene improves survival after ventricular fibrillation by mitigating impaired calcium handling in animal models. Circulation 129, 875–885 (2014).
Lowery, C. M. Use of stored implanted cardiac defibrillator electrograms in catheter ablation of ventricular fibrillation. Pacing Clin. Electrophysiol. 36, 76–85 (2013).
Xiao, L. et al. Unique cardiac Purkinje fiber transient outward current β-subunit composition: a potential molecular link to idiopathic ventricular fibrillation. Circ. Res. 112, 1310–1322 (2013).
Marsman, R. F. et al. A mutation in CALM1 encoding calmodulin in familial idiopathic ventricular fibrillation in childhood and adolescence. J. Am. Coll. Cardiol. 3, 259–266 (2014).
Cheung, J. W. et al. Short-coupled polymorphic ventricular tachycardia at rest linked to a novel ryanodine receptor (RyR2) mutation: leaky RyR2 channels under non-stress conditions. Int. J. Cardiol. 180, 228–236 (2015).
Tamargo, J., Caballero, R., Gomez, R., Valenzuela, C. & Delpon, E. Pharmacology of cardiac potassium channels. Cardiovasc. Res. 62, 9–33 (2004).
Caceres, J. et al. Sustained bundle branch reentry as a mechanism of clinical tachycardia. Circulation 79, 256–270 (1989).
Wissner, E. et al. Long-term outcome after catheter ablation for left posterior fascicular ventricular tachycardia without development of left posterior fascicular block. J. Cardiovasc. Electrophysiol. 23, 1179–1184 (2012).
Belhassen, B. & Viskin, S. Idiopathic ventricular tachycardia and fibrillation. J. Cardiovasc. Electrophysiol. 4, 356–368 (1993).
Bogun, F. et al. Role of Purkinje fibers in post-infarction ventricular tachycardia. J. Am. Coll. Cardiol. 48, 2500–2507 (2006).
Hayashi, M. et al. Novel mechanism of postinfarction ventricular tachycardia originating in surviving left posterior Purkinje fibers. Heart Rhythm 3, 908–918 (2006).
Bansch, D. et al. Successful catheter ablation of electrical storm after myocardial infarction. Circulation 108, 3011–3016 (2003).
Yokoshiki, H., Mitsuyama, H., Watanabe, M., Mizukami, K. & Tsutsui, H. Suppression of ventricular fibrillation by electrical modification of the Purkinje system in hypertrophic cardiomyopathy. Heart Vessels 29, 709–717 (2014).
Author information
Authors and Affiliations
Contributions
M. Haissaguerre, E.V., B.S., M. Hocini, and O.B. researched data for the article. M. Haissaguerre, E.V., B.S., and O.B. wrote the article. M. Haissaguerre and O.B. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Haissaguerre, M., Vigmond, E., Stuyvers, B. et al. Ventricular arrhythmias and the His–Purkinje system. Nat Rev Cardiol 13, 155–166 (2016). https://doi.org/10.1038/nrcardio.2015.193
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2015.193
This article is cited by
-
Enhanced optimization-based method for the generation of patient-specific models of Purkinje networks
Scientific Reports (2023)
-
Mapping and ablation of ventricular fibrillation substrate
Journal of Interventional Cardiac Electrophysiology (2023)
-
An Inverse Problem Involving a Viscous Eikonal Equation with Applications in Electrophysiology
Vietnam Journal of Mathematics (2022)
-
Catheter ablation of short-coupled variant of torsade de pointes
Clinical Research in Cardiology (2022)
-
Advances in Mapping and Ablation of Ventricular Fibrillation
Current Treatment Options in Cardiovascular Medicine (2021)