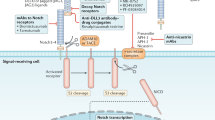
Abstract
Tumor relapse and metastasis remain major obstacles for improving overall cancer survival, which may be due at least in part to the existence of cancer stem cells (CSCs). CSCs are characterized by tumorigenic properties and the ability to self-renew, form differentiated progeny, and develop resistance to therapy. CSCs use many of the same signaling pathways that are found in normal stem cells, such as Wnt, Notch, and Hedgehog (Hh). The origin of CSCs is not fully understood, but data suggest that they originate from normal stem or progenitor cells, or possibly other cancer cells. Therapeutic targeting of both CSCs and bulk tumor populations may provide a strategy to suppress tumor regrowth. Development of agents that target critical steps in the Wnt, Notch, and Hh pathways will be complicated by signaling cross-talk. The role that embryonic signaling pathways play in the function of CSCs, the development of new anti-CSC therapeutic agents, and the complexity of potential CSC signaling cross-talk are described in this Review.
Key Points
-
The stochastic model and cancer stem cell (CSC) model of tumorigenesis could be combined to help explain tumor relapse and metastasis
-
DNA mutations, microenvironmental factors, and/or epithelial-to-mesenchymal transition may drive CSCs towards a metastatic phenotype
-
Tumors composed of small populations of CSCs plus large numbers of bulk tumor cells may be particularly susceptible to combination drug regimens that target each cell population
-
The potential for cross-talk among signaling pathways by CSCs opens new opportunities for designing combination drug regimens
-
New experimental agents are being developed to block Wnt, Notch, and Hedgehog signaling by CSCs, some of which are being tested in early clinical trials
-
Measurement of biologic effects of anti-CSC therapeutic regimens will remain a challenge until more-effective methods to identify CSCs in vivo or in vitro surrogate assays are improved
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Dick, J. E. Stem cell concepts renew cancer research. Blood 112, 4793–4807 (2008).
Dick, J. E. Looking ahead in cancer stem cell research. Nat. Biotechnol. 27, 44–46 (2009).
Johnsen, H. E. et al. Cancer stem cells and the cellular hierarchy in haematological malignancies. Eur. J. Cancer 45 (Suppl. 1), 194–201 (2009).
Odoux, C. et al. A stochastic model for cancer stem cell origin in metastatic colon cancer. Cancer Res. 68, 6932–6941 (2008).
Frank, N. Y., Schatton, T. & Frank, M. H. The therapeutic promise of the cancer stem cell concept. J. Clin. Invest. 120, 41–50 (2010).
McGovern, M., Voutev, R., Maciejowski, J., Corsi, A. K. & Hubbard, E. J. A “latent niche” mechanism for tumor initiation. Proc. Natl Acad. Sci. USA 106, 11617–11622 (2009).
Peacock, C. D. et al. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc. Natl Acad. Sci. USA 104, 4048–4053 (2007).
Reya, T. & Clevers, H. Wnt signalling in stem cells and cancer. Nature 434, 843–850 (2005).
Brabletz, T., Jung, A., Spaderna, S., Hlubek, F. & Kirchner, T. Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat. Rev. Cancer 5, 744–749 (2005).
Quintana, E. et al. Efficient tumour formation by single human melanoma cells. Nature 456, 593–598 (2008).
Dieter, S. M. et al. Distinct types of human colon cancer initiating cells contribute to primary tumor and metastasis formation [abstract 9]. Proc. Am. Assoc. Cancer Res. (2010).
Schmidt, M. et al. Polyclonal long-term repopulating stem cell clones in a primate model. Blood 100, 2737–2743 (2002).
Lapidot, T. et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367, 645–648 (1994).
Bonnet, D. & Dick, J. E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 3, 730–737 (1997).
Al-Hajj, M. et al. Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. USA 100, 3983–3988 (2003).
Singh, S. K. et al. Identification of human brain tumour initiating cells. Nature 432, 396–401 (2004).
Dalerba, P. et al. Phenotypic characterization of human colorectal cancer stem cells. Proc. Natl Acad. Sci. USA 104, 10158–10163 (2007).
O'Brien, C. A., Pollett, A., Gallinger, S. & Dick, J. E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445, 106–110 (2007).
Ricci-Vitiani, L. et al. Identification and expansion of human colon-cancer-initiating cells. Nature 445, 111–115 (2007).
Prince, M. E. et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl Acad. Sci. USA 104, 973–978 (2007).
Hermann, P. C. et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 1, 313–323 (2007).
Li, C. et al. Identification of pancreatic cancer stem cells. Cancer Res. 67, 1030–1037 (2007).
Yamashita, T. et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology 136, 1012–1024 (2009).
Schatton, T. et al. Identification of cells initiating human melanomas. Nature 451, 345–349 (2008).
Yang, Z. F. et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell 13, 153–166 (2008).
Bertolini, G. et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc. Natl Acad. Sci. USA 106, 16281–16286 (2009).
Zhang, S. et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 68, 4311–4320 (2008).
Wang, X. et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 461, 495–500 (2009).
Chan, K. S. et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc. Natl Acad. Sci. USA 106, 14016–14021 (2009).
Suvà, M. L. et al. Identification of cancer stem cells in Ewing's sarcoma. Cancer Res. 69, 1776–1781 (2009).
Huang, E. H. et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 69, 3382–3389 (2009).
Wang, Z. et al. Down-regulation of notch-1 inhibits invasion by inactivation of nuclear factor-kappaB, vascular endothelial growth factor, and matrix metalloproteinase-9 in pancreatic cancer cells. Cancer Res. 66, 2778–2784 (2006).
Krivtsov, A. V. et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature 442, 818–822 (2006).
Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. Stem cells, cancer, and cancer stem cells. Nature 414, 105–111 (2001).
Jamieson, C. H. et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N. Engl. J. Med. 351, 657–667 (2004).
Artavanis-Tsakonas, S., Rand, M. D. & Lake, R. J. Notch signaling: cell fate control and signal integration in development. Science 284, 770–776 (1999).
Dontu, G. et al. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 6, R605–R615 (2004).
Androutsellis-Theotokis, A. et al. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature 442, 823–826 (2006).
Rampal, R., Arboleda-Velasquez, J. F., Nita-Lazar, A., Kosik, K. S. & Haltiwanger, R. S. Highly conserved O-fucose sites have distinct effects on Notch1 function. J. Biol. Chem. 280, 32133–32140 (2005).
Gordon, W. R. et al. Structural basis for autoinhibition of Notch. Nat. Struct. Mol. Biol. 14, 295–300 (2007).
Real, P. J. et al. Gamma-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia. Nat. Med. 15, 50–58 (2009).
Rizzo, P. et al. Rational targeting of Notch signaling in cancer. Oncogene 27, 5124–5131 (2008).
Duarte, A. et al. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. 18, 2474–2478 (2004).
Suchting, S. et al. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc. Natl Acad. Sci. USA 104, 3225–3230 (2007).
Ridgway, J. et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature 444, 1083–1087 (2006).
Yan, M. et al. Chronic DLL4 blockade induces vascular neoplasms. Nature 463, E6–E7 (2010).
Moellering, R. E. et al. Direct inhibition of the NOTCH transcription factor complex. Nature 462, 182–188 (2009).
Sawyer, T. K. AILERON Therapeutics. Chem. Biol. Drug Des. 73, 3–6 (2009).
Epenetos, A., Kousparou, C. & Stylianou, S. Inhibition of Notch signaling for the treatment of human carcinomas. AACR Meeting Abstracts A5502 (2009).
Wu, Y. et al. Therapeutic antibody targeting of individual Notch receptors. Nature 464, 1052–1057 (2010).
Ingham, P. W. & McMahon, A. P. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15, 3059–3087 (2001).
Hahn, H. et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell 85, 841–851 (1996).
Johnson, R. L. et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science 272, 1668–1671 (1996).
Yauch, R. L. et al. A paracrine requirement for hedgehog signalling in cancer. Nature 455, 406–410 (2008).
Micchelli, C. A., The, I., Selva, E., Mogila, V. & Perrimon, N. Rasp, a putative transmembrane acyltransferase, is required for Hedgehog signaling. Development 129, 843–851 (2002).
Ruiz i Altaba, A., Mas, C. & Stecca, B. The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends Cell Biol. 17, 438–447 (2007).
Molofsky, A. V. et al. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 425, 962–967 (2003).
Adesina, A. M. et al. Gene expression profiling reveals signatures characterizing histologic subtypes of hepatoblastoma and global deregulation in cell growth and survival pathways. Hum. Pathol. 40, 843–853 (2009).
Zhao, C. et al. Hedgehog signaling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature 458, 776–779 (2009).
Feldmann, G. et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 67, 2187–2196 (2007).
Taipale, J. et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature 406, 1005–1009 (2000).
Kiselyov, A. S. Targeting the hedgehog signaling pathway with small molecules. Anticancer Agents Med. Chem. 6, 445–449 (2006).
Rubin, L. L. & de Sauvage, F. J. Targeting the Hedgehog pathway in cancer. Nat. Rev. Drug Discov. 5, 1026–1033 (2006).
Stanton, B. Z. et al. A small molecule that binds Hedgehog and blocks its signaling in human cells. Nat. Chem. Biol. 5, 154–156 (2009).
Hyman, J. M. et al. Small-molecule inhibitors reveal multiple strategies for Hedgehog pathway blockade. Proc. Natl Acad. Sci. USA 106, 14132–14137 (2009).
Olive, K. P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009).
LoRusso, P. M. et al. A first-in-human, first-in-class, phase (ph) I study of systemic Hedgehog (Hh) pathway antagonist, GDC-0449, in patients (pts) with advanced solid tumors [abstract]. J. Clin. Oncol. 26, a3516 (2008).
Von Hoff, D. D. et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N. Engl. J. Med. 361, 1164–1172 (2009).
Angers, S. & Moon, R. T. Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 10, 468–477 (2009).
Grigoryan, T., Wend, P., Klaus, A. & Birchmeier, W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 22, 2308–2341 (2008).
Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 127, 469–480 (2006).
Andrade, A. C., Nilsson, O., Barnes, K. M. & Baron, J. Wnt gene expression in the post-natal growth plate: regulation with chondrocyte differentiation. Bone 40, 1361–1369 (2007).
Takada, R. et al. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev. Cell 11, 791–801 (2006).
Ching, W. & Nusse, R. A dedicated Wnt secretion factor. Cell 125, 432–433 (2006).
Hsieh, J. C., Rattner, A., Smallwood, P. M. & Nathans, J. Biochemical characterization of Wnt-frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proc. Natl Acad. Sci. USA 96, 3546–3551 (1999).
Schulte, G. & Bryja, V. The Frizzled family of unconventional G-protein-coupled receptors. Trends Pharmacol. Sci. 28, 518–525 (2007).
de La Coste, A. et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc. Natl Acad. Sci. USA 95, 8847–8851 (1998).
Kim, M. S., Kim, S. S., Ahn, C. H., Yoo, N. J. & Lee, S. H. Frameshift mutations of Wnt pathway genes AXIN2 and TCF7L2 in gastric carcinomas with high microsatellite instability. Hum. Pathol. 40, 58–64 (2009).
Koesters, R. et al. Mutational activation of the beta-catenin proto-oncogene is a common event in the development of Wilms' tumors. Cancer Res. 59, 3880–3882 (1999).
Martin, V. et al. Epigenetic regulation of the non-canonical Wnt pathway in acute myeloid leukemia. Cancer Sci. 101, 425–432 (2010).
Malanchi, I. et al. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature 452, 650–653 (2008).
Majeti, R. et al. Dysregulated gene expression networks in human acute myelogenous leukemia stem cells. Proc. Natl Acad. Sci. USA 106, 3396–3401 (2009).
Müller-Tidow, C. et al. Translocation products in acute myeloid leukemia activate the Wnt signaling pathway in hematopoietic cells. Mol. Cell Biol. 24, 2890–2904 (2004).
Chen, B. et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 5, 100–107 (2009).
Emami, K. H. et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected]. Proc. Natl Acad. Sci. USA 101, 12682–12687 (2004).
Takahashi-Yanaga, F. & Kahn, M. Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clin. Cancer Res. 16, 3153–3162 (2010).
Fujii, N. et al. An antagonist of dishevelled protein-protein interaction suppresses beta-catenin-dependent tumor cell growth. Cancer Res. 67, 573–579 (2007).
Shan, J., Shi, D. L., Wang, J. & Zheng, J. Identification of a specific inhibitor of the dishevelled PDZ domain. Biochemistry 44, 15495–15503 (2005).
He, B. et al. Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene 24, 3054–3058 (2005).
You, L. et al. An anti-Wnt-2 monoclonal antibody induces apoptosis in malignant melanoma cells and inhibits tumor growth. Cancer Res. 64, 5385–5389 (2004).
Thiery, J. P., Acloque, H., Huang, R. Y. & Nieto, M. A. Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 (2009).
Shook, D. & Keller, R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech. Dev. 120, 1351–1383 (2003).
Kim, M. A. et al. Prognostic importance of epithelial-mesenchymal transition-related protein expression in gastric carcinoma. Histopathology 54, 442–451 (2009).
Mareel, M. et al. E-cadherin/catenin/cytoskeleton complex: a regulator of cancer invasion. J. Cell Physiol. 173, 271–274 (1997).
Dissanayake, S. K. et al. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J. Biol. Chem. 282, 17259–17271 (2007).
Vincan, E. & Barker, N. The upstream components of the Wnt signalling pathway in the dynamic EMT and MET associated with colorectal cancer progression. Clin. Exp. Metastasis 25, 657–663 (2008).
Massagué, J. TGFbeta in cancer. Cell 134, 215–230 (2008).
Yang, J. & Weinberg, R. A. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell 14, 818–829 (2008).
Bailey, J. M., Singh, P. K. & Hollingsworth, M. A. Cancer metastasis facilitated by developmental pathways: Sonic hedgehog, Notch, and bone morphogenic proteins. J. Cell Biochem. 102, 829–839 (2007).
Li, X. et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J. Natl Cancer Inst. 100, 672–679 (2008).
Frank, N. Y. et al. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 65, 4320–4333 (2005).
Ambudkar, S. V., Kimchi-Sarfaty, C., Sauna, Z. E. & Gottesman, M. M. P-glycoprotein: from genomics to mechanism. Oncogene 22, 7468–7485 (2003).
Diehn, M. et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 458, 780–783 (2009).
Itasaki, N. & Hoppler, S. Crosstalk between Wnt and bone morphogenic protein signaling: a turbulent relationship. Dev. Dyn. 239, 16–33 (2010).
Sun, L., Tian, Z. & Wang, J. A direct cross-talk between interferon-gamma and sonic hedgehog signaling that leads to the proliferation of neuronal precursor cells. Brain Behav. Immun. 24, 220–228 (2010).
Vivekanand, P. & Rebay, I. Intersection of signal transduction pathways and development. Annu. Rev. Genet. 40, 139–157 (2006).
Ma, J. et al. Mammalian target of rapamycin regulates murine and human cell differentiation through STAT3/p63/Jagged/Notch cascade. J. Clin. Invest. 120, 103–114 (2010).
Wang, Z. et al. Down-regulation of Notch-1 and Jagged-1 inhibits prostate cancer cell growth, migration and invasion, and induces apoptosis via inactivation of Akt, mTOR, and NF-kappaB signaling pathways. J. Cell. Biochem. 109, 726–736 (2010).
Meurette, O. et al. Notch activation induces Akt signaling via an autocrine loop to prevent apoptosis in breast epithelial cells. Cancer Res. 69, 5015–5022 (2009).
Dai, J. et al. Cross-talk between Notch and EGFR signaling in human breast cancer cells. Cancer Invest. 27, 533–540 (2009).
Purow, B. W. et al. Notch-1 regulates transcription of the epidermal growth factor receptor through p53. Carcinogenesis 29, 918–925 (2008).
Deb, S. P., Muñoz, R. M., Brown, D. R., Subler, M. A. & Deb, S. Wild-type human p53 activates the human epidermal growth factor receptor promoter. Oncogene 9, 1341–1349 (1994).
Mellinghoff, I. K. et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N. Engl. J. Med. 353, 2012–2024 (2005).
Osipo, C. et al. ErbB-2 inhibition activates Notch-1 and sensitizes breast cancer cells to a gamma-secretase inhibitor. Oncogene 27, 5019–5032 (2008).
Hirose, H. et al. Notch pathway as candidate therapeutic target in Her2/Neu/ErbB2 receptor-negative breast tumors. Oncol. Rep. 23, 35–43 (2010).
Guo, X. & Wang, X. F. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 19, 71–88 (2009).
Zavadil, J., Cermak, L., Soto-Nieves, N. & Böttinger, E. P. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 23, 1155–1165 (2004).
He, J. et al. Suppressing Wnt signaling by the hedgehog pathway through sFRP-1. J. Biol. Chem. 281, 35598–35602 (2006).
Bhagwandin, V. J. & Shay. J. W. Pancreatic cancer stem cells: fact or fiction? Biochim. Biophys. Acta 1792, 248–259 (2009).
Deangelo, D. J. et al. A phase I clinical trial of the notch inhibitor MK-0752 in patients with T-cell acute lymphoblastic leukemia/lymphoma (T-ALL) and other leukemias [abstract]. J. Clin. Oncol. 24, a6585 (2006).
Peters, J. U. et al. Novel orally active, dibenzazepinone-based gamma-secretase inhibitors. Bioorg Med. Chem. Lett. 17, 5918–5923 (2007).
Kopan, R. & Ilagan, M. X. Gamma-secretase: proteasome of the membrane? Nat. Rev. Mol. Cell Biol. 5, 499–504 (2004).
Li, K. et al. Modulation of Notch signaling by antibodies specific for the extracellular negative regulatory region of NOTCH3. J. Biol. Chem. 283, 8046–8054 (2006).
Hoey, T. et al. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell 5, 168–177 (2009).
Noguera-Troise, I. et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 444, 1032–1037 (2006).
Garcés, C. et al. Notch-1 controls the expression of fatty acid-activated transcription factors and is required for adipogenesis. J. Biol. Chem. 272, 29729–29734 (1997).
Nickoloff, B. J. et al. Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kappaB and PPARgamma. Cell Death Differ. 9, 842–855 (2002).
Rudin, C. M., Hann, C. L., Peacock, C. D. & Watkins, D. N. Novel systemic therapies for small cell lung cancer. J. Natl Compr. Canc. Netw. 6, 315–322 (2008).
Barginear, M. F., Leung, M. & Budman, D. R. The hedgehog pathway as a therapeutic target for treatment of breast cancer. Breast Cancer Res. Treat. 116, 239–246 (2009).
Fukukawa, C. Katagiri, T., Nakatsuru, S. & Nakamura, Y. Therapeutic potential of antibodies against frizzled homologue 10, a cell-surface protein, for synovial sarcoma [abstract]. Proc. Amer. Assoc. Cancer Res. 47, 1975 (2006).
Yoshizumi, T. et al. Thiazolidinedione, a peroxisome proliferator-activated receptor-gamma ligand, inhibits growth and metastasis of HT-29 human colon cancer cells through differentiation-promoting effects. Int. J. Oncol. 25, 631–639 (2004).
Arber, N. et al. Celecoxib for the prevention of colorectal adenomatous polyps. N. Engl. J. Med. 355, 885–895 (2006).
van Stolk, R. et al. Phase I trial of exisulind (sulindac sulfone, FGN-1) as a chemopreventive agent in patients with familial adenomatous polyposis. Clin. Cancer Res. 6, 78–89 (2000).
Cong, F., Zhang, J., Pao, W., Zhou, P. & Varmus, H. A protein knockdown strategy to study the function of beta-catenin in tumorigenesis. BMC Mol. Biol. 4, 10 (2003).
Liu, J., Stevens, J., Matsunami, N. & White, R. L. Targeted degradation of beta-catenin by chimeric F-box fusion proteins. Biochem. Biophys. Res. Commun. 313, 1023–1029 (2004).
Nagao, R. et al. A novel β-catenin inhibitor, AV65 suppresses the growth of CML cell lines which acquire imatinib-resistance because of Abl kinase domain mutations including T315I and hypoxia-adaptation. 50th ASH Annual Meeting and Exposition Abstracts, 1081 (2008).
Su, Y., Ishikawa, S., Kojima, M. & Liu, B. Eradication of pathogenic beta-catenin by Skp1/Cullin/F box ubiquitination machinery. Proc. Natl Acad. Sci. USA 100, 12729–12734 (2003).
Author information
Authors and Affiliations
Contributions
N. Takebe, P. J. Harris, R. Q. Warren and S. P. Ivy contributed equally to the literature review, discussions of the content, writing and reviewing and editing of this manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J. Cortés is a consultant for Roche. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Takebe, N., Harris, P., Warren, R. et al. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol 8, 97–106 (2011). https://doi.org/10.1038/nrclinonc.2010.196
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2010.196
This article is cited by
-
WNT5A regulates the proliferation, apoptosis and stemness of human stem Leydig cells via the β-catenin signaling pathway
Cellular and Molecular Life Sciences (2024)
-
Unravelling the enigma of siRNA and aptamer mediated therapies against pancreatic cancer
Molecular Cancer (2023)
-
Proteome-wide mendelian randomization study implicates therapeutic targets in common cancers
Journal of Translational Medicine (2023)
-
HERC2 promotes inflammation-driven cancer stemness and immune evasion in hepatocellular carcinoma by activating STAT3 pathway
Journal of Experimental & Clinical Cancer Research (2023)
-
Intervening in hnRNPA2B1-mediated exosomal transfer of tumor-suppressive miR-184-3p for tumor microenvironment regulation and cancer therapy
Journal of Nanobiotechnology (2023)