Key Points
-
Chemoprevention is a developing supplemental approach to promote cancer prevention, especially in high-risk populations
-
Studying commonly used drugs as candidate cancer chemopreventive agents has the advantage of usually having a low-risk profile and is associated with much clinical experience
-
Statins, bisphosphonates and metformin have all shown a promising degree of anticancer activity in several tumour sites
-
Evidence accumulated thus far is mostly mechanistic or from association studies in humans; data from randomized controlled trials are still lacking
-
The use of these agents for cancer chemoprevention is not yet formally indicated; however, it could be considered in high-risk populations especially if supporting pharmacogenetic markers of efficacy are available
Abstract
Cancer risk reduction using pharmacological means is an attractive modern preventive approach that supplements the classical behavioural prevention recommendations. Medications that are commonly used by large populations to treat a variety of common, non–cancer–related, medical situations are an attractive candidate pool. This Review discusses three pharmacological agents with the most evidence for their potential as cancer chemopreventive agents: anti–hypercholesterolaemia medications (statins), an antidiabetic agent (metformin) and antiosteoporosis drugs (bisphosphonates). Data are accumulating to support a significant negative association of certain statins with cancer occurrence or survival in several major tumour sites (mostly gastrointestinal tumours and breast cancer), with an augmented combined effect with aspirin or other non–steroidal anti–inflammatory drugs. Metformin, but not other hypoglycaemic drugs, also seems to have some antitumour growth activity, but the amount of evidence in human studies, mainly in breast cancer, is still limited. Experimental and observational data have identified bisphosphonates as a pharmacological group that could have significant impact on incidence and mortality of more than one subsite of malignancy. At the current level of evidence these potential chemopreventive drugs should be considered in high–risk situations or using the personalized approach of maximizing individual benefits and minimizing the potential for adverse effects with the aid of pharmacogenetic indicators.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cuzick, J. et al. Preventive therapy for breast cancer: a consensus statement. Lancet Oncol. 12, 496–503 (2011).
Thun, M. J., Jacobs, E. J. & Patrono, C. The role of aspirin in cancer prevention. Nat. Rev. Clin. Oncol. 9, 259–267 (2012).
Cuzick, J. et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 10, 501–507 (2009).
Rennert, G., Rennert, H. S., Pinchev, M. & Gruber, S. B. A case-control study of levothyroxine and the risk of colorectal cancer. J. Natl Cancer Inst. 102, 568–572 (2010).
Arrieta, O. et al. Colchicine delays the development of hepatocellular carcinoma in patients with hepatitis virus-related liver cirrhosis. Cancer 107, 1852–1858 (2006).
Yasuda, T. et al. Anti-gout agent allopurinol exerts cytotoxicity to human hormone-refractory prostate cancer cells in combination with tumor necrosis factor-related apoptosis-inducing ligand. Mol. Cancer Res. 6, 1852–1860 (2008).
Umar, A., Dunn, B. K. & Greenwald, P. Future directions in cancer prevention. Nat. Rev. Cancer 12, 835–848 (2012).
Sperati, F. et al. Vitamin D supplementation and breast cancer prevention: a systematic review and meta-analysis of randomized clinical trials. PLoS ONE 8, e69269 (2013).
Murphy, M. S. & O'Brien, T. in Pharmacology and Therapeutics: principles to practice Ch. 23 (eds Waldman, S. A. & Terzic, A.) 303–320 (Elsevier, Amsterdam, 2009).
Law, M. & Rudnicka, A. R. Statin safety: a systematic review. Am. J. Cardiol. 97, 52C–60C (2006).
Gazzerro, P. et al. Pharmacological actions of statins: a critical appraisal in the management of cancer. Pharmacol. Rev. 64, 102–146 (2012).
Jain, M. K. & Ridker, P. M. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat. Rev. Drug Discov. 4, 977–987 (2005).
Kwak, B., Mulhaupt, F., Myit, S. & Mach, F. Statins as a newly recognized type of immunomodulator. Nat. Med. 6, 1399–1402 (2000).
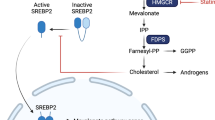
Goldstein, J. L. & Brown, M. S. Regulation of the mevalonate pathway. 343, 425–430 (1990).
Clendening, J. W. et al. Dysregulation of the mevalonate pathway promotes transformation. Proc. Natl Acad. Sci. USA 107, 15051–15056 (2010).
Inano, H., Suzuki, K., Onoda, M. & Wakabayashi, K. Anti-carcinogenic activity of simvastatin during the promotion phase of radiation-induced mammary tumorigenesis of rats. Carcinogenesis 18, 1723–1727 (1997).
Alonso, D. F. et al. Reduction of mouse mammary tumor formation and metastasis by lovastatin, an inhibitor of the mevalonate pathway of cholesterol synthesis. Breast Cancer Res. Treat. 50, 83–93 (1998).
Campbell, M. J. et al. Breast cancer growth prevention by statins. Cancer Res. 66, 8707–8714 (2006).
Kubatka, P. et al. Immunohistochemical and histomorphological analysis of rat mammary tumors after simvastatin treatment. Neoplasma 59, 516–523 (2012).
Yu, X. et al. BRCA1 overexpression sensitizes cancer cells to lovastatin via regulation of cyclin D1-CDK4-p21WAF1/CIP1 pathway: analyses using a breast cancer cell line and tumoral xenograft model. Int. J. Oncol. 33, 555–563 (2008).
Hawk, M. A., Cesen, K. T., Siglin, J. C., Stoner, G. D. & Ruch, R. J. Inhibition of lung tumor cell growth in vitro and mouse lung tumor formation by lovastatin. Cancer Lett. 109, 217–222 (1996).
Swamy, M. V. et al. Chemoprevention of familial adenomatous polyposis by low doses of atorvastatin and celecoxib given individually and in combination to APCM in mice. Cancer Res. 66, 7370–7377 (2006).
Suh, N. et al. Combination of atorvastatin with sulindac or naproxen profoundly inhibits colonic adenocarcinomas by suppressing the p65/β-catenin/cyclin D1 signaling pathway in rats. Cancer Prev. Res. (Phila.) 4, 1895–1902 (2011).
Cho, S. J. et al. Simvastatin induces apoptosis in human colon cancer cells and in tumor xenografts, and attenuates colitis-associated colon cancer in mice. Int. J. Cancer 123, 951–957 (2008).
Kodach, L. L. et al. The effect of statins in colorectal cancer is mediated through the bone morphogenetic protein pathway. Gastroenterology 133, 1272–1281 (2007).
Mohammed, A. et al. Atorvastatin delays progression of pancreatic lesions to carcinoma by regulating PI3/AKT signaling in p48Cre/+ LSL-KrasG12D/+ mice. Int. J. Cancer 131, 1951–1962 (2012).
Sumi, S. et al. Inhibition of pancreatic adenocarcinoma cell growth by lovastatin. Gastroenterology 103, 982–989 (1992).
Gbelcová, H. et al. Differences in antitumor effects of various statins on human pancreatic cancer. Int. J. Cancer 122, 1214–1221 (2008).
Björkhem-Bergman, L., Acimovic, J., Torndal, U. B., Parini, P. & Eriksson, L. C. Lovastatin prevents carcinogenesis in a rat model for liver cancer. Effects of ubiquinone supplementation. Anticancer Res. 30, 1105–1112 (2010).
Acimovic, J., Lövgren-Sandblom, A., Eriksson, L. C. & Björkhem-Bergman, L. The anti-carcinogenic effect of statins in a rat model correlates with levels and synthesis of ubiquinone. Biochem. Biophys. Res. Commun. 425, 348–352 (2012).
Taras, D. et al. Pravastatin reduces lung metastasis of rat hepatocellular carcinoma via a coordinated decrease of MMP expression and activity. J. Hepatol. 46, 69–76 (2007).
Cao, Z. et al. MYC phosphorylation, activation, and tumorigenic potential in hepatocellular carcinoma are regulated by HMG-CoA reductase. Cancer Res. 71, 2286–2297 (2011).
Matsuura, M. et al. Statin-mediated reduction of osteopontin expression induces apoptosis and cell growth arrest in ovarian clear cell carcinoma. Oncol. Rep. 25, 41–47 (2011).
Kochuparambil, S. T., Al-Husein, B., Goc, A., Soliman, S. & Somanath, P. R. Anticancer efficacy of simvastatin on prostate cancer cells and tumor xenografts is associated with inhibition of Akt and reduced prostate-specific antigen expression. J. Pharmacol. Exp. Ther. 336, 496–505 (2011).
Takwi, A. A. et al. A statin-regulated microRNA represses human c-Myc expression and function. EMBO Mol. Med. 4, 896–909 (2012).
Kidera, Y. et al. Reduction of lung metastasis, cell invasion, and adhesion in mouse melanoma by statin-induced blockade of the Rho/Rho-associated coiled-coil-containing protein kinase pathway. J. Exp. Clin. Cancer Res. 29, 127 (2010).
[No authors listed] Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N. Engl. J. Med. 339, 1349–1357 (1998).
Shepherd, J. et al. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 360, 1623–1630 (2002).
Bjerre, L. M. & LeLorier, J. Do statins cause cancer? A meta-analysis of large randomized clinical trials. Am. J. Med. 110, 716–723 (2001).
Jacobs, E. J., Newton, C. C., Thun, M. J. & Gapstur, S. M. Long-term use of cholesterol-lowering drugs and cancer incidence in a large United States cohort. Cancer Res. 71, 1763–1771 (2011).
Friis, S. et al. Cancer risk among statin users: a population-based cohort study. Int. J. Cancer 114, 643–647 (2005).
Farwell, W. R. et al. The association between statins and cancer incidence in a veterans population. J. Natl Cancer Inst. 100, 134–139 (2008).
Haukka, J. et al. Incidence of cancer and statin usage--record linkage study. Int. J. Cancer 126, 279–284 (2010).
Fröhlich, G. M. et al. Statins and the risk of cancer after heart transplantation. Circulation 126, 440–447 (2012).
Nielsen, S. F., Nordestgaard, B. G. & Bojesen, S. E. Statin use and reduced cancer-related mortality. N. Engl. J. Med. 367, 1792–1802 (2012).
Blais, L., Desgagné, A. & LeLorier, J. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the risk of cancer: a nested case-control study. Arch. Intern. Med. 160, 2363–2368 (2000).
Graaf, M. R., Beiderbeck, A. B., Egberts, A. C., Richel, D. J. & Guchelaar, H. J. The risk of cancer in users of statins. J. Clin. Oncol. 22, 2388–2394 (2004).
Cauley, J. A. et al. Lipid-lowering drug use and breast cancer in older women: a prospective study. J. Womens Health (Larchmt) 12, 749–756 (2003).
Cauley, J. A. et al. Statin use and breast cancer: prospective results from the Women's Health Initiative. J. Natl Cancer Inst. 98, 700–707 (2006).
Kwan, M. L., Habel, L. A., Flick, E. D., Quesenberry, C. P. & Caan, B. Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors. Breast Cancer Res. Treat. 109, 573–579 (2008).
Ahern, T. P. et al. Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J. Natl Cancer Inst. 103, 1461–1468 (2011).
Boudreau, D. M. et al. The association between 3-hydroxy-3-methylglutaryl conenzyme A inhibitor use and breast carcinoma risk among postmenopausal women: a case-control study. Cancer 100, 2308–2316 (2004).
Lee, J. E. et al. Statin use and colorectal cancer risk according to molecular subtypes in two large prospective cohort studies. Cancer Prev. Res. (Phila.) 4, 1808–1815 (2011).
Simon, M. S. et al. Prospective analysis of association between use of statins or other lipid-lowering agents and colorectal cancer risk. Ann. Epidemiol. 22, 17–27 (2012).
Poynter, J. N. et al. Statins and the risk of colorectal cancer. N. Engl. J. Med. 352, 2184–2192 (2005).
Samadder, N. J. et al. Risk of colorectal cancer in self-reported inflammatory bowel disease and modification of risk by statin and NSAID use. Cancer 117, 1640–1648 (2011).
Vinogradova, Y., Hippisley-Cox, J., Coupland, C. & Logan, R. F. Risk of colorectal cancer in patients prescribed statins, nonsteroidal anti-inflammatory drugs, and cyclooxygenase-2 inhibitors: nested case-control study. Gastroenterology 133, 393–402 (2007).
Hoffmeister, M., Chang-Claude, J. & Brenner, H. Individual and joint use of statins and low-dose aspirin and risk of colorectal cancer: a population-based case-control study. Int. J. Cancer 121, 1325–1330 (2007).
Hachem, C., Morgan, R., Johnson, M., Kuebeler, M. & El-Serag, H. Statins and the risk of colorectal carcinoma: a nested case-control study in veterans with diabetes. Am. J. Gastroenterol. 104, 1241–1248 (2009).
Robertson, D. J. et al. Neither long-term statin use nor atherosclerotic disease is associated with risk of colorectal cancer. Clin. Gastroenterol. Hepatol. 8, 1056–1061 (2010).
Broughton, T., Sington, J. & Beales, I. L. Statin use is associated with a reduced incidence of colorectal cancer: a colonoscopy-controlled case-control study. BMC Gastroenterol. 12, 36 (2012).
Lakha, F. et al. Statin use and association with colorectal cancer survival and risk: case control study with prescription data linkage. BMC Cancer 12, 487 (2012).
Broughton, T., Sington, J. & Beales, I. L. Statin use is associated with a reduced incidence of colorectal adenomatous polyps. Int. J. Colorectal Dis. 28, 469–476 (2013).
Kawata, S. et al. Effect of pravastatin on survival in patients with advanced hepatocellular carcinoma. A randomized controlled trial. Br. J. Cancer 84, 886–891 (2001).
Kastelein, F. et al. Nonsteroidal anti-inflammatory drugs and statins have chemopreventative effects in patients with Barrett's esophagus. Gastroenterology 141, 2000–2008 (2011).
Kantor, E. D., Onstad, L., Blount, P. L., Reid, B. J. & Vaughan, T. L. Use of statin medications and risk of esophageal adenocarcinoma in persons with Barrett's esophagus. Cancer Epidemiol. Biomarkers Prev. 21, 456–461 (2012).
Liu, W., Choueiri, T. K. & Cho, E. Statin use and the risk of renal cell carcinoma in 2 prospective US cohorts. Cancer 118, 797–803 (2012).
Tsan, Y. T., Lee, C. H., Wang, J. D. & Chen, P. C. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J. Clin. Oncol. 30, 623–630 (2012).
Khurana, V., Bejjanki, H. R., Caldito, G. & Owens, M. W. Statins reduce the risk of lung cancer in humans: a large case-control study of US veterans. Chest 131, 1282–1288 (2007).
Nguyen, D. M., Richardson, P. & El-Serag, H. B. Medications (NSAIDs, statins, proton pump inhibitors) and the risk of esophageal adenocarcinoma in patients with Barrett's esophagus. Gastroenterology 138, 2260–2266 (2010).
Beales, I. L., Vardi, I., Dearman, L. & Broughton, T. Statin use is associated with a reduction in the incidence of esophageal adenocarcinoma: a case control study. Dis. Esophagus http://dx.doi.org/10.1111/j.1442–2050201201412x.
Chiu, H. F., Ho, S. C., Chang, C. C., Wu, T. N. & Yang, C. Y. Statins are associated with a reduced risk of gastric cancer: a population-based case-control study. Am. J. Gastroenterol. 106, 2098–2103 (2011).
Khurana, V., Sheth, A., Caldito, G. & Barkin, J. S. Statins reduce the risk of pancreatic cancer in humans: a case-control study of half a million veterans. Pancreas 34, 260–265 (2007).
El-Serag, H. B., Johnson, M. L., Hachem, C. & Morgana, R. O. Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Gastroenterology 136, 1601–1608 (2009).
Chiu, H. F., Ho, S. C., Chen, C. C. & Yang, C. Y. Statin use and the risk of liver cancer: a population-based case–control study. Am. J. Gastroenterol. 106, 894–898 (2011).
Kaye, J. A. & Jick, H. Statin use and cancer risk in the General Practice Research Database. Br. J. Cancer 90, 635–637 (2004).
Setoguchi, S., Glynn, R. J., Avorn, J., Mogun, H. & Schneeweiss, S. Statins and the risk of lung, breast, and colorectal cancer in the elderly. Circulation 115, 27–33 (2007).
Smeeth, L., Douglas, I., Hall, A. J., Hubbard, R. & Evans, S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br. J. Clin. Pharmacol. 67, 99–109 (2009).
Friedman, G. D. et al. Screening statins for possible carcinogenic risk: up to 9 years of follow-up of 361,859 recipients. Pharmacoepidemiol. Drug Saf. 17, 27–36 (2008).
Marelli, C. et al. Statins and risk of cancer: a retrospective cohort analysis of 45,857 matched pairs from an electronic medical records database of 11 million adult Americans. J. Am. Coll. Cardiol. 58, 530–537 (2011).
Vinogradova, Y., Coupland, C. & Hippisley-Cox, J. Exposure to statins and risk of common cancers: a series of nested case-control studies. BMC Cancer 11, 409 (2011).
Boudreau, D. M., Yu, O., Miglioretti, D. L., Buist, D. S., Heckbert, S. R. & Daling, J. R. Statin use and breast cancer risk in a large population-based setting. Cancer Epidemiol. Biomarkers Prev. 16, 416–421 (2007).
Kaye, J. A., Meier, C. R., Walker, A. M. & Jick, H. Statin use, hyperlipidaemia, and the risk of breast cancer. Br. J. Cancer 86, 1436–1439 (2002).
Woditschka, S., Habel, L. A., Udaltsova, N., Friedman, G. D. & Sieh, W. Lipophilic statin use and risk of breast cancer subtypes. Cancer Epidemiol. Biomarkers Prev. 19, 2479–2487 (2010).
Jacobs, E. J. et al. Cholesterol-lowering drugs and colorectal cancer incidence in a large United States cohort. J. Natl Cancer Inst. 98, 69–72 (2006).
Ng, K. et al. Relationship between statin use and colon cancer recurrence and survival: results from CALGB 89803. J. Natl Cancer Inst. 103, 1540–1551 (2011).
Bertagnolli, M. M. et al. Statin use and colorectal adenoma risk: results from the adenoma prevention with celecoxib trial. Cancer Prev. Res. (Phila.) 3, 588–596 (2010).
Coogan, P. F., Smith, J. & Rosenberg, L. Statin use and risk of colorectal cancer. J. Natl Cancer Inst. 99, 32–40 (2007).
Yang, Y. X. et al. Chronic statin therapy and the risk of colorectal cancer. Pharmacoepidemiol. Drug Saf. 17, 869–876 (2008).
Cheng, M. H. et al. Statin use and the risk of colorectal cancer: a population-based case-control study. World J. Gastroenterol. 17, 5197–5202 (2011).
Bradley, M. C., Hughes, C. M., Cantwell, M. M. & Murray, L. J. Statins and pancreatic cancer risk: a nested case-control study. Cancer Causes Control 21, 2093–2100 (2010).
Yu, O., Boudreau, D. M., Buist, D. S. & Miglioretti, D. L. Statin use and female reproductive organ cancer risk in a large population-based setting. Cancer Causes Control 20, 609–616 (2009).
Sacks, F. M. et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N. Engl. J. Med. 335, 1001–1009 (1996).
ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA 288, 2998–3007 (2002).
Pedersen, T. R. et al. Follow-up study of patients randomized in the Scandinavian simvastatin survival study (4S) of cholesterol lowering. Am. J. Cardiol. 86, 257–262 (2000).
Strandberg, T. E. et al. Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S). Lancet 364, 771–777 (2004).
Bulbulia, R. et al. Effects on 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20,536 high-risk individuals: a randomised controlled trial. Lancet 378, 2013–2020 (2011).
Suissa, S., Dell'aniello, S., Vahey, S. & Renoux, C. Time-window bias in case-control studies: statins and lung cancer. Epidemiology 22, 228–231 (2011).
Notarnicola, M. et al. Up-regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in left-sided human colon cancer. Anticancer Res. 24, 3837–3842 (2004).
Sharad, S. et al. Prostate cancer gene expression signature of patients with high body mass index. Prostate Cancer Prostatic Dis. 14, 22–29 (2011).
Higgins, M. J. et al. A short-term biomarker modulation study of simvastatin in women at increased risk of a new breast cancer. Breast Cancer Res. Treat. 131, 915–924 (2012).
Dale, K. M., Coleman, C. I., Henyan, N. N., Kluger, J. & White, C. M. Statins and cancer risk: a meta-analysis. JAMA 295, 74–80 (2006).
Kuoppala, J., Lamminpää, A. & Pukkala, E. Statins and cancer: A systematic review and meta-analysis. Eur. J. Cancer 44, 2122–2132 (2008).
Taylor, M. L., Wells, B. J. & Smolak, M. J. Statins and cancer: a meta-analysis of case-control studies. Eur. J. Cancer Prev. 17, 259–268 (2008).
Bonovas, S., Filioussi, K., Flordellis, C. S. & Sitaras, N. M. Statins and the risk of colorectal cancer: a meta-analysis of 18 studies involving more than 1.5 million patients. J. Clin. Oncol. 25, 3462–3468 (2007).
Cui, X. et al. Statin use and risk of pancreatic cancer: a meta-analysis. Cancer Causes Control 23, 1099–1111 (2012).
Singh, S., Singh, P. P., Singh, A. G., Murad, M. H. & Sanchez, W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology 144, 323–332 (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Reiner, Z. Statins in the primary prevention of cardiovascular disease. Nat. Rev. Cardiol. 10, 453–464 (2013).
Lipkin, S. M. et al. Genetic variation in 3-hydroxy-3-methylglutaryl CoA reductase modifies the chemopreventive activity of statins for colorectal cancer. Cancer Prev. Res. (Phila.) 3, 597–603 (2010).
Bailey, K. M. et al. Hepatic metabolism and transporter gene variants enhance response to rosuvastatin in patients with acute myocardial infarction: the GEOSTAT-1 Study. Circ. Cardiovasc. Genet. 3, 276–285 (2010).
Brautbar, A. et al. Variants in the APOA5 gene region and the response to combination therapy with statins and fenofibric acid in a randomized clinical trial of individuals with mixed dyslipidemia. Atherosclerosis 219, 737–742 (2011).
Peters, B. J. et al. Pharmacogenetic interactions between ABCB1 and SLCO1B1 tagging SNPs and the effectiveness of statins in the prevention of myocardial infarction. Pharmacogenomics 11, 1065–1076 (2010).
Chasman, D. I. et al. Genetic determinants of statin-induced low-density lipoprotein cholesterol reduction: the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. Circ. Cardiovasc. Genet. 5, 257–264 (2012).
Freed-Pastor, W. A. et al. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell 148, 244–258 (2012).
Power, D. G., Gloglowski, E. & Lipkin, S. M. Clinical genetics of hereditary colorectal cancer. Hematol. Oncol. Clin. North Am. 24, 837–859 (2010).
Rizza, R. A. & Vella, A. in Pharmacology and Therapeutics: principles to practice Ch. 37 (eds Waldman, S. A. & Terzic, A.) 557–570 (Elsevier, Amsterdam, 2009).
Xu, J. et al. Insulin enhances growth hormone induction of the MEK/ERK signaling pathway. J. Biol. Chem. 281, 982–992 (2006).
Bennett, W. L., Keeton, A. B., Ji, S., Xu, J. & Messina, J. L. Insulin regulation of growth hormone receptor gene expression: involvement of both the PI-3 kinase and MEK/ERK signaling pathways. Endocrine 32, 219–226 (2007).
Melander, O. et al. Plasma proneurotensin and incidence of diabetes, cardiovascular disease, breast cancer, and mortality. JAMA 308, 1469–1475 (2012).
Zhou, G. et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 (2001).
Shaw, R. J. et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310, 1642–1646 (2005).
Hemminki, A. et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature 391, 184–187 (1998).
Zakikhani, M., Dowling, R., Fantus, I. G., Sonenberg, N. & Pollak, M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 66, 10269–10273 (2006).
Zakikhani, M., Dowling, R. J., Sonenberg, N. & Pollak, M. N. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer Prev. Res. (Phila.) 1, 369–375 (2008).
Ben Sahra, I. et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 71, 4366–4372 (2011).
Deng, X. S. et al. Metformin targets Stat3 to inhibit cell growth and induce apoptosis in triple-negative breast cancers. Cell Cycle 11, 367–376 (2012).
Oliveras-Ferraros, C., Vazquez-Martin, A. & Menendez, J. A. Genome-wide inhibitory impact of the AMPK activator metformin on [kinesins, tubulins, histones, auroras and polo-like kinases] M-phase cell cycle genes in human breast cancer cells. Cell Cycle 8, 1633–1636 (2009).
Cufí, S. et al. Metformin lowers the threshold for stress-induced senescence: a role for the microRNA-200 family and miR-205. Cell Cycle 11, 1235–1246 (2012).
Zhuang, Y. & Miskimins, W. K. Metformin induces both caspase-dependent and poly(ADP-ribose) polymerase-dependent cell death in breast cancer cells. Mol. Cancer Res. 9, 603–615 (2011).
Hirsch, H. A., Iliopoulos, D., Tsichlis, P. N. & Struhl, K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 69, 7507–7511 (2009).
Oliveras-Ferraros, C. et al. Micro(mi)RNA expression profile of breast cancer epithelial cells treated with the anti-diabetic drug metformin: induction of the tumor suppressor miRNA let-7a and suppression of the TGFβ-induced oncomiR miRNA-181a. Cell Cycle 10, 1144–1151 (2011).
Buzzai, M. et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 67, 6745–6752 (2007).
Memmott, R. M. et al. Metformin prevents tobacco carcinogen--induced lung tumorigenesis. Cancer Prev. Res. (Phila.) 3, 1066–1076 (2010).
Algire, C., Zakikhani, M., Blouin, M. J., Shuai, J. H. & Pollak, M. Metformin attenuates the stimulatory effect of a high-energy diet on in vivo LLC1 carcinoma growth. Endocr. Relat. Cancer 15, 833–839 (2008).
Schneider, M. B. et al. Prevention of pancreatic cancer induction in hamsters by metformin. Gastroenterology 120, 1263–1270 (2001).
Blandino, G. et al. Metformin elicits anticancer effects through the sequential modulation of DICER and c-MYC. Nat. Commun. 3, 865 (2012).
Rattan, R., Graham, R. P., Maguire, J. L., Giri, S. & Shridhar, V. Metformin suppresses ovarian cancer growth and metastasis with enhancement of cisplatin cytotoxicity in vivo. Neoplasia 13, 483–491 (2011).
Shank, J. J. et al. Metformin targets ovarian cancer stem cells in vitro and in vivo. Gynecol. Oncol. 127, 390–397 (2012).
Phoenix, K. N., Vumbaca, F. & Claffey, K. P. Therapeutic metformin/AMPK activation promotes the angiogenic phenotype in the ERα negative MDA-MB-435 breast cancer model. Breast Cancer Res. Treat. 113, 101–111 (2009).
Martin, M. J., Hayward, R., Viros, A. & Marais, R. Metformin accelerates the growth of BRAFV600E-driven melanoma by upregulating VEGF-A. Cancer Discov. 2, 344–355 (2012).
Larsson, S. C., Orsini, N. & Wolk, A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J. Natl Cancer Inst. 97, 1679–1687 (2005).
Michels, K. B. et al. Type 2 diabetes and subsequent incidence of breast cancer in the Nurses' Health Study. Diabetes Care 26, 1752–1758 (2003).
Redaniel, M. T., Jeffreys, M., May, M. T., Ben-Shlomo, Y. & Martin, R. M. Associations of type 2 diabetes and diabetes treatment with breast cancer risk and mortality: a population-based cohort study among British women. Cancer Causes Control 23, 1785–1795 (2012).
Boyle, P. et al. Diabetes and breast cancer risk: a meta-analysis. Br. J. Cancer 107, 1608–1617 (2012).
Luo, J. et al. Diabetes and lung cancer among postmenopausal women. Diabetes Care 35, 1485–1491 (2012).
Newton, C. C., Gapstur, S. M., Campbell, P. T. & Jacobs, E. J. Type 2 diabetes mellitus, insulin use, and risk of bladder cancer in a large cohort study. Int. J. Cancer 132, 2186–2191 (2013).
Kasper, J. S. & Giovannucci, E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol. Biomarkers Prev. 15, 2056–2062 (2006).
Johnson, J. A. et al. Diabetes and cancer (1): evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologia 55, 1607–1618 (2012).
Decensi, A. et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev. Res. (Phila.) 3, 1451–1461 (2010).
Noto, H., Goto, A., Tsujimoto, T. & Noda, M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS ONE 7, e33411 (2012).
Zhang, Z. J. et al. Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes: a meta-analysis. Diabetes Care 34, 2323–2328 (2011).
Col, N. F., Ochs, L., Springmann, V., Aragaki, A. K. & Chlebowski, R. T. Metformin and breast cancer risk: a meta-analysis and critical literature review. Breast Cancer Res. Treat. 135, 639–646 (2012).
Suissa, S. & Azoulay, L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care 35, 2665–2673 (2012).
Home, P. D. et al. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 53, 1838–1845 (2010).
Bonanni, B. et al. Dual effect of metformin on breast cancer proliferation in a randomized presurgical trial. J. Clin. Oncol. 30, 2593–2600 (2012).
Niraula, S. et al. Metformin in early breast cancer: a prospective window of opportunity neoadjuvant study. Breast Cancer Res. Treat. 135, 821–830 (2012).
Hadad, S. et al. Evidence for biological effects of metformin in operable breast cancer: a pre-operative, window-of-opportunity, randomized trial. Breast Cancer Res. Treat. 128, 783–794 (2011).
Hosono, K. et al. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev. Res. (Phila.) 3, 1077–1083 (2010).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Higurashi, T. et al. Metformin efficacy and safety for colorectal polyps: a double-blind randomized controlled trial. BMC Cancer 12, 118 (2012).
Wang, D. S. et al. Involvement of organic cation transporter 1 in the hepatic and intestinal distribution of metformin. J. Pharmacol. Exp. Ther. 302, 510–515 (2002).
Shu, Y. et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J. Clin. Invest. 117, 1422–1431 (2007).
Zhou, K. et al. Common variants near ATM are associated with glycemic response to metformin in type 2 diabetes. Nat. Genet. 43, 117–120 (2011).
Clarke, L. C. & Khosla, S. in Pharmacology and Therapeutics: principles to practice Ch. 39 (eds Waldman, S. A. & Terzic, A.) 587–610 (Elsevier, Amsterdam, 2009).
Russell, R. G. Bisphosphonates: from bench to bedside. Ann. N. Y. Acad. Sci. 1068, 367–401 (2006).
Tang, X. et al. Bisphosphonates suppress insulin-like growth factor 1-induced angiogenesis via the HIF-1α/VEGF signaling pathways in human breast cancer cells. Int. J. Cancer 126, 90–103 (2010).
Senaratne, S. G., Mansi, J. L. & Colston, K. W. The bisphosphonate zoledronic acid impairs Ras membrane [correction of impairs membrane] localisation and induces cytochrome c release in breast cancer cells. Br. J. Cancer 86, 1479–1486 (2002).
Hiraga, T., Williams, P. J., Ueda, A., Tamura, D. & Yoneda, T. Zoledronic acid inhibits visceral metastases in the 4T1/luc mouse breast cancer model. Clin. Cancer Res. 10, 4559–4567 (2004).
Senaratne, S. G., Pirianov, G., Mansi, J. L., Arnett, T. R. & Colston, K. W. Bisphosphonates induce apoptosis in human breast cancer cell lines. Br. J. Cancer 82, 1459–1468 (2000).
Li, Y. Y. et al. Zoledronic acid is unable to induce apoptosis, but slows tumor growth and prolongs survival for non-small-cell lung cancers. Lung Cancer 59, 180–191 (2008).
Brown, H. K., Ottewell, P. D., Coleman, R. E. & Holen, I. The kinetochore protein Cenp-F is a potential novel target for zoledronic acid in breast cancer cells. J. Cell. Mol. Med. 15, 501–513 (2011).
Coscia, M. et al. Zoledronic acid repolarizes tumour-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. J. Cell. Mol. Med. 14, 2803–2815 (2010).
Melani, C., Sangaletti, S., Barazzetta, F. M., Werb, Z. & Colombo, M. P. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 67, 11438–11446 (2007).
Daubiné, F., Le Gall, C., Gasser, J., Green, J. & Clézardin, P. Antitumor effects of clinical dosing regimens of bisphosphonates in experimental breast cancer bone metastasis. J. Natl Cancer Inst. 99, 322–330 (2007).
Hashimoto, K. et al. Alendronate suppresses tumor angiogenesis by inhibiting Rho activation of endothelial cells. Biochem. Biophys. Res. Commun. 354, 478–484 (2007).
Santini, D. et al. Repeated intermittent low-dose therapy with zoledronic acid induces an early, sustained, and long-lasting decrease of peripheral vascular endothelial growth factor levels in cancer patients. Clin. Cancer Res. 13 (15 Pt 1), 4482–4486 (2007).
Santini, D. et al. In vivo effects of zoledronic acid on peripheral gammadelta T lymphocytes in early breast cancer patients. Cancer Immunol. Immunother. 58, 31–38 (2009).
Diel, I. J. et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N. Engl. J. Med. 339, 357–363 (1998).
Powles, T. et al. Randomized, placebo-controlled trial of clodronate in patients with primary operable breast cancer. J. Clin. Oncol. 20, 3219–3224 (2002).
Saarto, T., Vehmanen, L., Virkkunen, P. & Blomqvist, C. Ten-year follow-up of a randomized controlled trial of adjuvant clodronate treatment in node-positive breast cancer patients. Acta Oncol. 43, 650–656 (2004).
Kristensen, B. et al. Bisphosphonate treatment in primary breast cancer: results from a randomised comparison of oral pamidronate versus no pamidronate in patients with primary breast cancer. Acta Oncol. 47, 740–746 (2008).
Gnant, M. et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N. Engl. J. Med. 360, 679–691 (2009).
Gnant, M. et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 12, 631–641 (2011).
Aft, R. et al. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial. Lancet Oncol. 11, 421–428 (2010).
Coleman, R. E. et al. Breast-cancer adjuvant therapy with zoledronic acid. N. Engl. J. Med. 365, 1396–1405 (2011).
Coleman, R. E. et al. The effects of adding zoledronic acid to neoadjuvant chemotherapy on tumour response: exploratory evidence for direct anti-tumour activity in breast cancer. Br. J. Cancer 102, 1099–1105 (2010).
Aft, R. L., Naughton, M., Trinkaus, K. & Weilbaecher, K. Effect of (Neo)adjuvant zoledronic acid on disease-free and overall survival in clinical stage II/III breast cancer. Br. J. Cancer 107, 7–11 (2012).
Coleman, R. et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann. Oncol. 24, 398–405 (2013).
Paterson, A. H. et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol. 13, 734–742 (2012).
Newcomb, P. A., Trentham-Dietz, A. & Hampton, J. M. Bisphosphonates for osteoporosis treatment are associated with reduced breast cancer risk. Br. J. Cancer 102, 799–802 (2010).
Chlebowski, R. T. et al. Oral bisphosphonate use and breast cancer incidence in postmenopausal women. J. Clin. Oncol. 28, 3582–3590 (2010).
Rennert, G., Pinchev, M. & Rennert, H. S. Use of bisphosphonates and risk of postmenopausal breast cancer. J. Clin. Oncol. 28, 3577–3581 (2010).
Monsees, G. M., Malone, K. E., Tang, M. T., Newcomb, P. A. & Li, C. I. Bisphosphonate use after estrogen receptor-positive breast cancer and risk of contralateral breast cancer. J. Natl Cancer Inst. 103, 1752–1760 (2011).
Rennert, G., Pinchev, M., Rennert, H. S. & Gruber, S. B. Use of bisphosphonates and reduced risk of colorectal cancer. J. Clin. Oncol. 29, 1146–1150 (2011).
Pazianas, M., Abrahamsen, B., Eiken, P. A., Eastell, R. & Russell, R. G. Reduced colon cancer incidence and mortality in postmenopausal women treated with an oral bisphosphonate-Danish National Register Based Cohort Study. Osteoporos. Int. 23, 2693–2701 (2012).
Singh, H., Nugent, Z., Demers, A., Mahmud, S. & Bernstein, C. Exposure to bisphosphonates and risk of colorectal cancer: a population-based nested case-control study. Cancer 118, 1236–1243 (2012).
Khalili, H., Huang, E. S., Ogino, S., Fuchs, C. S. & Chan, A. T. A prospective study of bisphosphonate use and risk of colorectal cancer. J. Clin. Oncol. 30, 3229–3233 (2012).
Passarelli, M. N. et al. Oral bisphosphonate use and colorectal cancer incidence in the Women's Health Initiative. J. Bone Miner. Res. http://dx.doi.org/10.1002/jbmr.1930.
Mason, M. D. et al. Oral sodium clodronate for nonmetastatic prostate cancer--results of a randomized double-blind placebo-controlled trial: Medical Research Council PR04 (ISRCTN61384873). J. Natl Cancer Inst. 99, 765–776 (2007).
Green, J. et al. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. BMJ 341, c4444 (2010).
Cardwell, C. R., Abnet, C. C., Cantwell, M. M. & Murray, L. J. Exposure to oral bisphosphonates and risk of esophageal cancer. JAMA 304, 657–663 (2010).
Chiang, C. H. et al. Oral alendronate use and risk of cancer in postmenopausal women with osteoporosis: A nationwide study. J. Bone Miner. Res. 27, 1951–1958 (2012).
Cardwell, C. R. et al. Exposure to oral bisphosphonates and risk of cancer. Int. J. Cancer 131, E717–E725 (2012).
Liu, Y. et al. Bisphosphonate use and the risk of breast cancer: a meta-analysis of published literature. Clin. Breast Cancer 12, 276–281 (2012).
Thosani, N. et al. Reduced risk of colorectal cancer with use of oral bisphosphonates: a systematic review and meta-analysis. J. Clin. Oncol. 31, 623–630 (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Marini, F. & Brandi, M. L. Pharmacogenetics of osteoporosis. F1000 Biol. Rep. 2, 63 (2010).
Estrada, K. et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat. Genet. 44, 491–501 (2012).
Libby, G. et al. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 32, 1620–1625 (2009).
Currie, C. J., Poole, C. D. & Gale, E. A. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 52, 1766–1777 (2009).
Chlebowski, R. T. et al. Diabetes, metformin, and breast cancer in postmenopausal women. J. Clin. Oncol. 30, 2844–2852 (2012).
Jiralerspong, S. et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J. Clin. Oncol. 27, 3297–3302 (2009).
Bodmer, M., Meier, C., Krähenbühl, S., Jick, S. S. & Meier, C. R. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care 33, 1304–1308 (2010).
Bosco, J. L., Antonsen, S., Sørensen, H. T., Pedersen, L. & Lash, T. L. Metformin and incident breast cancer among diabetic women: a population-based case-control study in Denmark. Cancer Epidemiol. Biomarkers Prev. 20, 101–111 (2011).
Bodmer, M., Becker, C., Meier, C., Jick, S. S. & Meier, C. R. Use of metformin is not associated with a decreased risk of colorectal cancer: a case-control analysis. Cancer Epidemiol. Biomarkers Prev. 21, 280–286 (2012).
Bodmer, M., Becker, C., Jick, S. S. & Meier, C. R. Metformin does not alter the risk of lung cancer: A case-control analysis. Lung Cancer 78, 133–137 (2012).
Smiechowski, B. B., Azoulay, L., Yin, H., Pollak, M. N. & Suissa, S. The use of metformin and the incidence of lung cancer in patients with type 2 diabetes. Diabetes Care 36, 124–129 (2013).
Li, D., Yeung, S. C., Hassan, M. M., Konopleva, M. & Abbruzzese, J. L. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 137, 482–488 (2009).
Bodmer, M., Becker, C., Meier, C., Jick, S. S. & Meier, C. R. Use of antidiabetic agents and the risk of pancreatic cancer: a case-control analysis. Am. J. Gastroenterol. 107, 620–626 (2012).
Hassan, M. M. et al. Association of diabetes duration and diabetes treatment with the risk of epatocellular carcinoma. Cancer 116, 1938–1946 (2010).
Diel, I. J. et al. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: a long-term follow-up. Ann. Oncol. 19, 2007–2011 (2008).
Newcomb, P. A., Trentham-Dietz, A. & Hampton, J. M. Bisphosphonates for osteoporosis treatment are associated with reduced breast cancer risk. Br. J. Cancer 102, 799–802 (2010).
Author information
Authors and Affiliations
Contributions
Both authors researched data for article, made a substantial contribution to discussion of the content, and wrote and edited the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
Statins: clinical studies mostly evaluating cancer as a secondary outcome of interest beyond cardiovascular events, and registry-based studies (PDF 3633 kb)
Supplementary Table 2
In vitro mechanistic studies of possible modes of action of statins (PDF 1438 kb)
Rights and permissions
About this article
Cite this article
Gronich, N., Rennert, G. Beyond aspirin—cancer prevention with statins, metformin and bisphosphonates. Nat Rev Clin Oncol 10, 625–642 (2013). https://doi.org/10.1038/nrclinonc.2013.169
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2013.169
This article is cited by
-
Can statins lessen the burden of virus mediated cancers?
Infectious Agents and Cancer (2022)
-
Collateral responses to classical cytotoxic chemotherapies are heterogeneous and sensitivities are sparse
Scientific Reports (2022)
-
Population-wide impacts of aspirin, statins, and metformin use on prostate cancer incidence and mortality
Scientific Reports (2021)
-
YAP promotes sorafenib resistance in hepatocellular carcinoma by upregulating survivin
Cellular Oncology (2021)
-
Independent and joint cross-sectional associations of statin and metformin use with mammographic breast density
Breast Cancer Research (2020)