Key Points
-
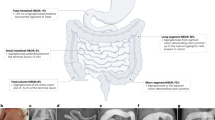
Hirschsprung disease is a potentially fatal birth defect in which the enteric nervous system (ENS) is missing from the distal bowel
-
Hirschsprung disease is a non-Mendelian, multigenic, partially penetrant, genetic disease
-
Surgery to bypass the aganglionic bowel is life-saving, but not curative
-
Enterocolitis occurs commonly even after surgery and can be life-threatening
-
Non-genetic factors affect ENS development, suggesting some cases of Hirschsprung disease might be preventable
-
Regenerative medicine strategies such as stem cell therapy could provide new treatment options
Abstract
Hirschsprung disease is defined by the absence of enteric neurons at the end of the bowel. The enteric nervous system (ENS) is the intrinsic nervous system of the bowel and regulates most aspects of bowel function. When the ENS is missing, there are no neurally mediated propulsive motility patterns, and the bowel remains contracted, causing functional obstruction. Symptoms of Hirschsprung disease include constipation, vomiting, abdominal distension and growth failure. Untreated disease usually causes death in childhood because bloodstream bacterial infections occur in the context of bowel inflammation (enterocolitis) or bowel perforation. Current treatment is surgical resection of the bowel to remove or bypass regions where the ENS is missing, but many children have problems after surgery. Although the anatomy of Hirschsprung disease is simple, many clinical features remain enigmatic, and diagnosis and management remain challenging. For example, the age of presentation and the type of symptoms that occur vary dramatically among patients, even though every affected child has missing neurons in the distal bowel at birth. In this Review, basic science discoveries are linked to clinical manifestations of Hirschsprung disease, including partial penetrance, enterocolitis and genetics. Insights into disease mechanisms that might lead to new prevention, diagnostic and treatment strategies are described.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hirschsprung, H. Stuhlträgheit neugeborener in folge von dilatation und hypertrophie des colons. Jahrbuch Kinderheilkunde Physische Erziehung (Berlin) 27, 1–7 (1888).
Corman, M. L. Classic articles in colonic and rectal surgery. Constipation in the newborn as a result of dilation and hypertrophy of the colon: Harald Hirschsprung, Jahrbuch für Kinderheilkunde, 1888. Dis. Colon Rectum 24, 408–410 (1981).
Furness, J. B., Callaghan, B. P., Rivera, L. R. & Cho, H. J. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv. Exp. Med. Biol. 817, 39–71 (2014).
Furness, J. B. The enteric nervous system. (Blackwell Publishing, 2006).
Odenwald, M. A. & Turner, J. R. The intestinal epithelial barrier: a therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 14, 9–21 (2017).
Knowles, C. H., Lindberg, G., Panza, E. & De Giorgio, R. New perspectives in the diagnosis and management of enteric neuropathies. Nat. Rev. Gastroenterol. Hepatol. 10, 206–218 (2013).
Furness, J. B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 9, 286–294 (2012).
Grubisic, V. & Gulbransen, B. D. Enteric glia: the most alimentary of all glia. J. Physiol. 595, 557–570 (2016).
Sharkey, K. A. Emerging roles for enteric glia in gastrointestinal disorders. J. Clin. Invest. 125, 918–925 (2015).
Furness, J. B. Integrated neural and endocrine control of gastrointestinal function. Adv. Exp. Med. Biol. 891, 159–173 (2016).
Vanner, S. et al. Fundamentals of neurogastroenterology: basic science. Gastroenterology 150, 1280–1291 (2016).
Hens, J., Vanderwinden, J. M., De Laet, M. H., Scheuermann, D. W. & Timmermans, J. P. Morphological and neurochemical identification of enteric neurones with mucosal projections in the human small intestine. J. Neurochem. 76, 464–471 (2001).
Brookes, S. J. Classes of enteric nerve cells in the guinea-pig small intestine. Anat. Rec. 262, 58–70 (2001).
Furness, J. B. Types of neurons in the enteric nervous system. J. Auton. Nerv. Syst. 81, 87–96 (2000).
Latorre, R., Sternini, C., De Giorgio, R. & Greenwood-Van Meerveld, B. Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol. Motil. 28, 620–630 (2016).
Psichas, A., Reimann, F. & Gribble, F. M. Gut chemosensing mechanisms. J. Clin. Invest. 125, 908–917 (2015).
Uesaka, T., Young, H. M., Pachnis, V. & Enomoto, H. Development of the intrinsic and extrinsic innervation of the gut. Dev. Biol. 417, 158–167 (2016).
Camilleri, M. & Di Lorenzo, C. The brain-gut axis: from basic understanding to treatment of irritable bowel syndrome and related disorders. J. Pediatr. Gastroenterol. Nutr. 54, 446–453 (2012).
Gonzalez-Arancibia, C. et al. What goes around comes around: novel pharmacological targets in the gut-brain axis. Therap Adv. Gastroenterol. 9, 339–353 (2016).
Carabotti, M., Scirocco, A., Maselli, M. A. & Severi, C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 28, 203–209 (2015).
Blair, P. J., Rhee, P. L., Sanders, K. M. & Ward, S. M. The significance of interstitial cells in neurogastroenterology. J. Neurogastroenterol. Motil. 20, 294–317 (2014).
Der-Silaphet, T., Malysz, J., Hagel, S., Arsenault, A. L. & Huizinga, J. D. Interstitial cells of Cajal direct normal propulsive contractile activity in the mouse small intestine. Gastroenterology 114, 724–736 (1998).
Spencer, N. J., Dinning, P. G., Brookes, S. J. & Costa, M. Insights into the mechanisms underlying colonic motor patterns. J. Physiol. 594, 4099–4116 (2016).
Mazet, B. Gastrointestinal motility and its enteric actors in mechanosensitivity: past and present. Pflugers Arch. 467, 191–200 (2015).
Heuckeroth, R. in Pediatric Neurogastroenterology (eds Faure, C., Di Lorenzo, C. & Thapar, N.) 291–302 (Springer, 2016).
McKeown, S. J., Stamp, L., Hao, M. M. & Young, H. M. Hirschsprung disease: a developmental disorder of the enteric nervous system. Wiley Interdiscip. Rev. Dev. Biol. 2, 113–129 (2013).
Amiel, J. et al. Hirschsprung disease, associated syndromes and genetics: a review. J. Med. Genet. 45, 1–14 (2008).
Skinner, M. Hirschsprung's disease. Curr. Probl. Surg. 33, 391–461 (1996).
Haricharan, R. N. & Georgeson, K. E. Hirschsprung disease. Semin. Pediatr. Surg. 17, 266–275 (2008).
Sherman, J. O. et al. A 40-year multinational retrospective study of 880 Swenson procedures. J. Pediatr. Surg. 24, 833–838 (1989).
Suita, S., Taguchi, T., Ieiri, S. & Nakatsuji, T. Hirschsprung's disease in Japan: analysis of 3852 patients based on a nationwide survey in 30 years. J. Pediatr. Surg. 40, 197–201 (2005).
Laughlin, D. M., Friedmacher, F. & Puri, P. Total colonic aganglionosis: a systematic review and meta-analysis of long-term clinical outcome. Pediatr. Surg. Int. 28, 773–779 (2012).
Reding, R. et al. Hirschsprung's disease: a 20-year experience. J. Pediatr. Surg. 32, 1221–1225 (1997).
Moore, S. W. & Zaahl, M. Clinical and genetic differences in total colonic aganglionosis in Hirschsprung's disease. J. Pediatr. Surg. 44, 1899–1903 (2009).
Swenson, O., Rheinlander, H. F. & Diamond, I. Hirschsprung's disease; a new concept of the etiology; operative results in 34 patients. N. Engl. J. Med. 241, 551–556 (1949).
Thakkar, H. S. et al. Functional outcomes in Hirschsprung disease: A single institution's 12-year experience. J. Pediatr. Surg. 52, 277–280 (2017).
Gosain, A. & Brinkman, A. S. Hirschsprung's associated enterocolitis. Curr. Opin. Pediatr. 27, 364–369 (2015).
Martucciello, G. Hirschsprung's disease, one of the most difficult diagnoses in pediatric surgery: a review of the problems from clinical practice to the bench. Eur. J. Pediatr. Surg. 18, 140–149 (2008).
Aboagye, J. et al. Age at presentation of common pediatric surgical conditions: reexamining dogma. J. Pediatr. Surg. 49, 995–999 (2014).
Menezes, M., Corbally, M. & Puri, P. Long-term results of bowel function after treatment for Hirschsprung's disease: a 29-year review. Pediatr. Surg. Int. 22, 987–990 (2006).
Doodnath, R. & Puri, P. A systematic review and meta-analysis of Hirschsprung's disease presenting after childhood. Pediatr. Surg. Int. 26, 1107–1110 (2010).
Surana, R., Quinn, F. M. J. & Puri, P. Neonatal intestinal perforation in Hirschsprung's disease. Pediatr. Surg. Int. 9, 501–502 (1994).
Bell, M. J. Perforation of the gastrointestinal tract and peritonitis in the neonate. Surg. Gynecol. Obstet. 160, 20–26 (1985).
Newman, B., Nussbaum, A. & Kirkpatrick, J. A. Jr. Bowel perforation in Hirschsprung's disease. AJR Am. J. Roentgenol 148, 1195–1197 (1987).
Hackam, D. J., Reblock, K. K., Redlinger, R. E. & Barksdale, E. M. Jr. Diagnosis and outcome of Hirschsprung's disease: does age really matter? Pediatr. Surg. Int. 20, 319–322 (2004).
Teitelbaum, D. H. et al. A decade of experience with the primary pull-through for hirschsprung disease in the newborn period: a multicenter analysis of outcomes. Ann. Surg. 232, 372–380 (2000).
Diamond, I. R., Casadiego, G., Traubici, J., Langer, J. C. & Wales, P. W. The contrast enema for Hirschsprung disease: predictors of a false-positive result. J. Pediatr. Surg. 42, 792–795 (2007).
Jung, P. M. Hirschsprung's disease: one surgeon's experience in one institution. J. Pediatr. Surg. 30, 646–651 (1995).
Rahman, N. et al. Rectal biopsy for Hirschsprung's disease—are we performing too many? Eur. J. Pediatr. Surg. 20, 95–97 (2010).
Downey, E. C., Hughes, E., Putnam, A. R., Baskin, H. J. & Rollins, M. D. Hirschsprung disease in the premature newborn: a population based study and 40-year single center experience. J. Pediatr. Surg. 50, 123–125 (2015).
Mugie, S. M., Benninga, M. A. & Di Lorenzo, C. Epidemiology of constipation in children and adults: a systematic review. Best Pract. Res. Clin. Gastroenterol. 25, 3–18 (2011).
Courdent, M., Beghin, L., Akré, J. & Turck, D. Infrequent stools in exclusively breastfed infants. Breastfeed. Med. 9, 442–445 (2014).
Mugie, S. M., Di Lorenzo, C. & Benninga, M. A. Constipation in childhood. Nat. Rev. Gastroenterol. Hepatol. 8, 502–511 (2011).
Frykman, P. K. & Short, S. S. Hirschsprung-associated enterocolitis: prevention and therapy. Semin. Pediatr. Surg. 21, 328–335 (2012).
Austin, K. M. The pathogenesis of Hirschsprung's disease-associated enterocolitis. Semin. Pediatr. Surg. 21, 319–327 (2012).
Demehri, F. R. et al. Altered fecal short chain fatty acid composition in children with a history of Hirschsprung-associated enterocolitis. J. Pediatr. Surg. 51, 81–86 (2016).
Pastor, A. C., Osman, F., Teitelbaum, D. H., Caty, M. G. & Langer, J. C. Development of a standardized definition for Hirschsprung's-associated enterocolitis: a Delphi analysis. J. Pediatr. Surg. 44, 251–256 (2009).
de Lorijn, F., Kremer, L. C., Reitsma, J. B. & Benninga, M. A. Diagnostic tests in Hirschsprung disease: a systematic review. J. Pediatr. Gastroenterol. Nutr. 42, 496–505 (2006).
Swenson, O. & Bill, A. H. Jr. Resection of rectum and rectosigmoid with preservation of the sphincter for benign spastic lesions producing megacolon; an experimental study. Surgery 24, 212–220 (1948).
De La Torre, L. & Langer, J. C. Transanal endorectal pull-through for Hirschsprung disease: technique, controversies, pearls, pitfalls, and an organized approach to the management of postoperative obstructive symptoms. Semin. Pediatr. Surg. 19, 96–106 (2010).
Dasgupta, R. & Langer, J. C. Hirschsprung disease. Curr. Probl. Surg. 41, 942–988 (2004).
Langer, J. C. Hirschsprung disease. Curr. Opin. Pediatr. 25, 368–374 (2013).
Swaminathan, M. & Kapur, R. P. Counting myenteric ganglion cells in histologic sections: an empirical approach. Hum. Pathol. 41, 1097–1108 (2010).
Soave, F. Hirschsprung's disease: a new surgical technique. Arch. Dis. Child 39, 116–124 (1964).
Duhamel, B. A new operation for the treatment of Hirschsprung's disease. Arch. Dis. Child 35, 38–39 (1960).
Zimmer, J., Tomuschat, C. & Puri, P. Long-term results of transanal pull-through for Hirschsprung's disease: a meta-analysis. Pediatr. Surg. Int. 32, 743–749 (2016).
Tomuschat, C., Zimmer, J. & Puri, P. Laparoscopic-assisted pull-through operation for Hirschsprung's disease: a systematic review and meta-analysis. Pediatr. Surg. Int. 32, 751–757 (2016).
Sieber, W. K. Hirschsprung's disease. Curr. Probl. Surg. 15, 1–76 (1978).
Ikeda, K. & Goto, S. Diagnosis and treatment of Hirschsprung's disease in Japan. An analysis of 1628 patients. Ann. Surg. 199, 400–405 (1984).
Rescorla, F. J., Morrison, A. M., Engles, D., West, K. W. & Grosfeld, J. L. Hirschsprung's disease. Evaluation of mortality and long-term function in 260 cases. Arch. Surg. 127, 934–941 (1992).
Pini Prato, A. et al. Hirschsprung's disease: what about mortality? Pediatr. Surg. Int. 27, 473–478 (2011).
Barnes, P. R., Lennard-Jones, J. E., Hawley, P. R. & Todd, I. P. Hirschsprung's disease and idiopathic megacolon in adults and adolescents. Gut 27, 534–541 (1986).
Bandre, E. et al. Hirschsprung's disease: management problem in a developing country. Afr. J. Paediatr. Surg. 7, 166–168 (2010).
Shinall, M. C. Jr, Koehler, E., Shyr, Y. & Lovvorn, H. N. 3rd. Comparing cost and complications of primary and staged surgical repair of neonatally diagnosed Hirschsprung's disease. J. Pediatr. Surg. 43, 2220–2225 (2008).
Kim, A. C. et al. Endorectal pull-through for Hirschsprung's disease-a multicenter, long-term comparison of results: transanal versus transabdominal approach. J. Pediatr. Surg. 45, 1213–1220 (2010).
Menezes, M. & Puri, P. Long-term outcome of patients with enterocolitis complicating Hirschsprung's disease. Pediatr. Surg. Int. 22, 316–318 (2006).
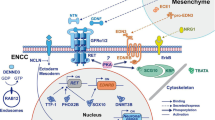
Heuckeroth, R. O. & Schafer, K. H. Gene-environment interactions and the enteric nervous system: Neural plasticity and Hirschsprung disease prevention. Dev. Biol. 417, 188–197 (2016).
Bondurand, N. & Southard-Smith, E. M. Mouse models of Hirschsprung disease and other developmental disorders of the enteric nervous system: old and new players. Dev. Biol. 417, 139–157 (2016).
Avetisyan, M., Schill, E. M. & Heuckeroth, R. O. Building a second brain in the bowel. J. Clin. Invest. 125, 899–907 (2015).
Lake, J. I. & Heuckeroth, R. O. Enteric nervous system development: migration, differentiation, and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G1–G24 (2013).
Goldstein, A. M., Hofstra, R. M. & Burns, A. J. Building a brain in the gut: development of the enteric nervous system. Clin. Genet. 83, 307–316 (2013).
Obermayr, F., Hotta, R., Enomoto, H. & Young, H. M. Development and developmental disorders of the enteric nervous system. Nat. Rev. Gastroenterol. Hepatol. 10, 43–57 (2012).
Lake, J. I., Tusheva, O. A., Graham, B. L. & Heuckeroth, R. O. Hirschsprung-like disease is exacerbated by reduced de novo GMP synthesis. J. Clin. Invest. 123, 4875–4887 (2013).
Newgreen, D. F., Dufour, S., Howard, M. J. & Landman, K. A. Simple rules for a “simple” nervous system? Molecular and biomathematical approaches to enteric nervous system formation and malformation. Dev. Biol. 382, 305–319 (2013).
Gui, H. et al. Whole exome sequencing coupled with unbiased functional analysis reveals new Hirschsprung disease genes. Genome Biol. 18, 48 (2017).
Kapoor, A. et al. Population variation in total genetic risk of hirschsprung disease from common RET, SEMA3 AND NRG1 susceptibility polymorphisms. Hum. Mol. Genet. 24, 2997–3003 (2015).
Luzon-Toro, B. et al. Exome sequencing reveals a high genetic heterogeneity on familial Hirschsprung disease. Sci. Rep. 5, 16473 (2015).
Bergeron, K. F., Silversides, D. W. & Pilon, N. The developmental genetics of Hirschsprung's disease. Clin. Genet. 83, 15–22 (2013).
Emison, E. S. et al. Differential contributions of rare and common, coding and noncoding Ret mutations to multifactorial Hirschsprung disease liability. Am. J. Hum. Genet. 87, 60–74 (2010).
Gabriel, S. B. et al. Segregation at three loci explains familial and population risk in Hirschsprung disease. Nat. Genet. 31, 89–93 (2002).
Alves, M. M. et al. Contribution of rare and common variants determine complex diseases-Hirschsprung disease as a model. Dev. Biol. 382, 320–329 (2013).
Pilon, N. Pigmentation-based insertional mutagenesis is a simple and potent screening approach for identifying neurocristopathy-associated genes in mice. Rare Dis. 4, e1156287 (2016).
Tang, W. et al. Exome-wide association study identified new risk loci for Hirschsprung's disease. Mol. Neurobiol. 54, 1777–1785 (2016).
Yang, J. et al. Exome sequencing identified NRG3 as a novel susceptible gene of Hirschsprung's disease in a Chinese population. Mol. Neurobiol. 47, 957–966 (2013).
Verma, A., Rattan, K. N. & Yadav, R. Neonatal intestinal obstruction: a 15 year experience in a tertiary care hospital. J. Clin. Diagn. Res. 10, SC10–SC13 (2016).
Badner, J. A., Sieber, W. K., Garver, K. L. & Chakravarti, A. A genetic study of Hirschsprung disease. Am. J. Hum. Genet. 46, 568–580 (1990).
Goldberg, E. L. An epidemiological study of Hirschsprung's disease. Int. J. Epidemiol. 13, 479–485 (1984).
Bodian, M. & Carter, O. O. A family study of Hirschsprung's disease. Ann. Hum. Genet. 26, 261–277 (1963).
Burzynski, G. M. et al. Identifying candidate Hirschsprung disease-associated RET variants. Am. J. Hum. Genet. 76, 850–858 (2005).
Emison, E. S. et al. A common sex-dependent mutation in a RET enhancer underlies Hirschsprung disease risk. Nature 434, 857–863 (2005).
Soret, R. et al. A collagen VI-dependent pathogenic mechanism for Hirschsprung's disease. J. Clin. Invest. 125, 4483–4496 (2015).
Attie, T. et al. Diversity of RET proto-oncogene mutations in familial and sporadic Hirschsprung disease. Hum. Mol. Genet. 4, 1381–1386 (1995).
Simkin, J. E., Zhang, D., Rollo, B. N. & Newgreen, D. F. Retinoic acid upregulates ret and induces chain migration and population expansion in vagal neural crest cells to colonise the embryonic gut. PLoS ONE 8, e64077 (2013).
Lang, D. et al. Pax3 is required for enteric ganglia formation and functions with Sox10 to modulate expression of c-Ret. J. Clin. Invest. 106, 963–971 (2000).
Pattyn, A., Morin, X., Cremer, H., Goridis, C. & Brunet, J.-F. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature 399, 366–370 (1999).
Southard-Smith, E. M., Kos, L. & Pavan, W. J. Sox10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nat. Genet. 18, 60–64 (1998).
Garcia-Barcelo, M. et al. Association study of PHOX2B as a candidate gene for Hirschsprung's disease. Gut 52, 563–567 (2003).
Li, Y. et al. SRY interference of normal regulation of the RET gene suggests a potential role of the Y-chromosome gene in sexual dimorphism in Hirschsprung disease. Hum. Mol. Genet. 24, 685–697 (2015).
Vohra, B. P. et al. Reduced endothelin converting enzyme-1 and endothelin-3 mRNA in the developing bowel of male mice may increase expressivity and penetrance of Hirschsprung disease-like distal intestinal aganglionosis. Dev. Dyn. 236, 106–117 (2007).
Fu, M. et al. Vitamin A facilitates enteric nervous system precursor migration by reducing Pten accumulation. Development 137, 631–640 (2010).
Saavedra, A., Baltazar, G. & Duarte, E. P. Driving GDNF expression: the green and the red traffic lights. Prog. Neurobiol. 86, 186–215 (2008).
Korbel, J. O. et al. The genetic architecture of Down syndrome phenotypes revealed by high-resolution analysis of human segmental trisomies. Proc. Natl Acad. Sci. USA 106, 12031–12036 (2009).
McCallion, A. S., Stames, E., Conlon, R. A. & Chakravarti, A. Phenotype variation in two-locus mouse models of Hirschsprung disease: tissue-specific interaction between Ret and Ednrb. Proc. Natl Acad. Sci. USA 100, 1826–1831 (2003).
Cantrell, V. A. et al. Interactions between Sox10 and EdnrB modulate penetrance and severity of aganglionosis in the Sox10Dom mouse model of Hirschsprung disease. Hum. Mol. Genet. 13, 2289–2301 (2004).
Stanchina, L. et al. Interactions between Sox10, Edn3 and Ednrb during enteric nervous system and melanocyte development. Dev. Biol. 295, 232–249 (2006).
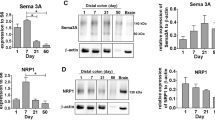
Schill, E. M. et al. Ibuprofen slows migration and inhibits bowel colonization by enteric nervous system precursors in zebrafish, chick and mouse. Dev. Biol. 409, 473–488 (2016).
Arnold, S. et al. Interaction between a chromosome 10 RET enhancer and chromosome 21 in the Down syndrome-Hirschsprung disease association. Hum. Mutat. 30, 771–775 (2009).
Jiang, Q. et al. Functional loss of semaphorin 3C and/or semaphorin 3D and their epistatic interaction with ret are critical to Hirschsprung disease liability. Am. J. Hum. Genet. 96, 581–596 (2015).
Carrasquillo, M. M. et al. Genome-wide association study and mouse model identify interaction between RET and EDNRB pathways in Hirschsprung disease. Nat. Genet. 32, 237–244 (2002).
Tam, P. K. Hirschsprung's disease: a bridge for science and surgery. J. Pediatr. Surg. 51, 18–22 (2016).
Duess, J. W. & Puri, P. Syndromic Hirschsprung's disease and associated congenital heart disease: a systematic review. Pediatr. Surg. Int. 31, 781–785 (2015).
Sarioglu, A., Tanyel, F. C., Buyukpamukcu, N. & Hicsonmez, A. Hirschsprung-associated congenital anomalies. Eur. J. Pediatr. Surg. 7, 331–337 (1997).
Hofmann, A. D., Duess, J. W. & Puri, P. Congenital anomalies of the kidney and urinary tract (CAKUT) associated with Hirschsprung's disease: a systematic review. Pediatr. Surg. Int. 30, 757–761 (2014).
Skinner, M. A., Safford, S. D., Reeves, J. G., Jackson, M. E. & Freemerman, A. J. Renal aplasia in humans is associated with RET mutations. Am. J. Hum. Genet. 82, 344–351 (2008).
Sampson, M. G. et al. Evidence for a recurrent microdeletion at chromosome 16p11.2 associated with congenital anomalies of the kidney and urinary tract (CAKUT) and Hirschsprung disease. Am. J. Med. Genet. A 152A, 2618–2622 (2010).
Pingault, V. et al. SOX10 mutations in patients with Waardenburg-Hirschsprung disease. Nat. Genet. 18, 171–173 (1998).
Bondurand, N. et al. A molecular analysis of the yemenite deaf-blind hypopigmentation syndrome: SOX10 dysfunction causes different neurocristopathies. Hum. Mol. Genet. 8, 1785–1789 (1999).
Parthey, K. et al. SOX10 mutation with peripheral amyelination and developmental disturbance of axons. Muscle Nerve 45, 284–290 (2012).
Matera, I. et al. PHOX2B mutations and polyalanine expansions correlate with the severity of the respiratory phenotype and associated symptoms in both congenital and late onset Central Hypoventilation syndrome. J. Med. Genet. 41, 373–380 (2004).
Makitie, O. & Kostjukovits, S. in GeneReviews® (eds Pagon, R. A. et al.) (University of Washington, Seattle, 1993).
Takahashi, M. et al. Co-segregation of MEN2 and Hirschsprung's disease: the same mutation of RET with both gain and loss-of-function? Hum. Mutat. 13, 331–336 (1999).
Moore, S. W. & Zaahl, M. G. Chasing the ubiquitous RET proto-oncogene in South African MEN2 families — implications for the surgeon. S. Afr. J. Surg. 48, 127–131 (2010).
Cohen, M. S. et al. Gastrointestinal manifestations of multiple endocrine neoplasia type 2. Ann. Surg. 235, 648–654 (2002).
Grey, W. et al. The RET E616Q variant is a gain of function mutation present in a family with features of multiple endocrine neoplasia 2A. Endocr. Pathol. 28, 41–48 (2017).
Arighi, E. et al. Biological effects of the dual phenotypic Janus mutation of ret cosegregating with both multiple endocrine neoplasia type 2 and Hirschsprung's disease. Mol. Endocrinol. 18, 1004–1017 (2004).
Luzon-Toro, B. et al. Next-generation-based targeted sequencing as an efficient tool for the study of the genetic background in Hirschsprung patients. BMC Med. Genet. 16, 89 (2015).
So, M. T. et al. RET mutational spectrum in Hirschsprung disease: evaluation of 601 Chinese patients. PLoS ONE 6, e28986 (2011).
Seri, M. et al. Frequency of RET mutations in long- and short-segment Hirschsprung disease. Hum. Mutat. 9, 243–249 (1997).
Owens, S. E. et al. Genome-wide linkage identifies novel modifier loci of aganglionosis in the Sox10Dom model of Hirschsprung disease. Hum. Mol. Genet. 14, 1549–1558 (2005).
Huang, J. et al. Genetic variation in the GDNF promoter affects its expression and modifies the severity of Hirschsprung's disease (HSCR) in rats carrying Ednrbsl mutations. Gene 575, 144–148 (2016).
Fattahi, F. et al. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature 531, 105–109 (2016).
Williams, J. et al. Updated estimates of neural tube defects prevented by mandatory folic acid fortification — United States, 1995–2011. MMWR Morb. Mortal. Wkly Rep. 64, 1–5 (2015).
Force, U. S. P. S. T. et al. Folic acid supplementation for the prevention of neural tube defects: US preventive services task force recommendation statement. JAMA 317, 183–189 (2017).
Rhoads, G. G. & Mills, J. L. Can vitamin supplements prevent neural tube defects? Current evidence and ongoing investigations. Clin. Obstet. Gynecol. 29, 569–579 (1986).
West, K. P. Jr. Extent of vitamin A deficiency among preschool children and women of reproductive age. J. Nutr. 132, 2857S–2866S (2002).
Mills, J. L., Simpson, J. L., Cunningham, G. C., Conley, M. R. & Rhoads, G. G. Vitamin A and birth defects. Am. J. Obstet. Gynecol. 177, 31–36 (1997).
Anderka, M. T., Lin, A. E., Abuelo, D. N., Mitchell, A. A. & Rasmussen, S. A. Reviewing the evidence for mycophenolate mofetil as a new teratogen: case report and review of the literature. Am. J. Med. Genet. A149A, 1241–1248 (2009).
Landman, K. A., Simpson, M. J. & Newgreen, D. F. Mathematical and experimental insights into the development of the enteric nervous system and Hirschsprung's disease. Dev. Growth Differ. 49, 277–286 (2007).
Sharma, D., Shastri, S. & Sharma, P. Intrauterine growth restriction: antenatal and postnatal aspects. Clin. Med. Insights Pediatr. 10, 67–83 (2016).
American Congress of Obstetricians and Gynecologists. Reducing risks of birth defects. ACOG https://www.acog.org/Patients/FAQs/Reducing-Risks-of-Birth-Defects#pregnancy (2017).
Musser, M. A., Correa, H. & Southard-Smith, E. M. Enteric neuron imbalance and proximal dysmotility in ganglionated intestine of the Sox10Dom/+ Hirschsprung mouse model. Cell. Mol. Gastroenterol. Hepatol. 1, 87–101 (2015).
Jain, S. et al. Mice expressing a dominant-negative Ret mutation phenocopy human Hirschsprung disease and delineate a direct role of Ret in spermatogenesis. Development 131, 5503–5513 (2004).
Gosain, A. Established and emerging concepts in Hirschsprung's-associated enterocolitis. Pediatr. Surg. Int. 32, 313–320 (2016).
Diebel, L. N., Dulchavsky, S. A. & Wilson, R. F. Effect of increased intra-abdominal pressure on mesenteric arterial and intestinal mucosal blood flow. J. Trauma 33, 45–49 (1992).
Pratap, A. et al. Doppler study of splanchnic hemodynamics in Hirschsprung's disease. Eur. Surg. Res. 39, 148–152 (2007).
Soeda, J., O'Briain, D. S. & Puri, P. Regional reduction in intestinal neuroendocrine cell populations in enterocolitis complicating Hirschsprung's disease. J. Pediatr. Surg. 28, 1063–1068 (1993).
Thiagarajah, J. R. et al. Altered goblet cell differentiation and surface mucus properties in Hirschsprung disease. PLoS ONE 9, e99944 (2014).
Sharkey, K. A. & Savidge, T. C. Role of enteric neurotransmission in host defense and protection of the gastrointestinal tract. Auton. Neurosci. 181, 94–106 (2014).
Hitch, M. C. et al. Ret heterozygous mice have enhanced intestinal adaptation after massive small bowel resection. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G1143–G1150 (2012).
Ibiza, S. et al. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature 535, 440–443 (2016).
Gross, E. R. et al. Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology 143, 408–417.e2 (2012).
Avetisyan, M. et al. Hepatocyte growth factor and MET Support mouse enteric nervous system development, the peristaltic response, and intestinal epithelial proliferation in response to injury. J. Neurosci. 35, 11543–11558 (2015).
Bjerknes, M. & Cheng, H. Modulation of specific intestinal epithelial progenitors by enteric neurons. Proc. Natl Acad. Sci. USA 98, 12497–12502 (2001).
Bush, T. G. et al. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell 93, 189–201 (1998).
Meir, M. et al. The glial cell-line derived neurotrophic factor: a novel regulator of intestinal barrier function in health and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G1118–G1123 (2016).
Brun, P. et al. Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system. Gastroenterology 145, 1323–1333 (2013).
Gershon, M. D. Serotonin is a sword and a shield of the bowel: serotonin plays offense and defense. Trans. Am. Clin. Climatol. Associ. 123, 268–280 (2012).
Rao, M. et al. Enteric glia regulate gastrointestinal motility but are not required for maintenance of the epithelium in mice. Gastroenterology 153, 1068–1081.e7 (2017).
Frykman, P. K. et al. Characterization of bacterial and fungal microbiome in children with Hirschsprung disease with and without a history of enterocolitis: a multicenter study. PLoS ONE 10, e0124172 (2015).
Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., Jansson, J. K. & Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230 (2012).
Hyland, N. P. & Cryan, J. F. Microbe-host interactions: influence of the gut microbiota on the enteric nervous system. Dev. Biol. 417, 182–187 (2016).
McVey Neufeld, K. A., Mao, Y. K., Bienenstock, J., Foster, J. A. & Kunze, W. A. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol. Motil. 25, 183–e88 (2013).
Obata, Y. & Pachnis, V. The effect of microbiota and the immune system on the development and organization of the enteric nervous system. Gastroenterology 151, 836–844 (2016).
Wang, X., Li, Z., Xu, Z., Wang, Z. & Feng, J. Probiotics prevent Hirschsprung's disease-associated enterocolitis: a prospective multicenter randomized controlled trial. Int. J. Colorectal Dis. 30, 105–110 (2015).
El-Sawaf, M., Siddiqui, S., Mahmoud, M., Drongowski, R. & Teitelbaum, D. H. Probiotic prophylaxis after pullthrough for Hirschsprung disease to reduce incidence of enterocolitis: A prospective, randomized, double-blind, placebo-controlled, multicenter trial. J. Pediatr. Surg. 48, 111–117 (2013).
Margolis, K. G., Gershon, M. D. & Bogunovic, M. Cellular organization of neuroimmune interactions in the gastrointestinal tract. Trends Immunol. 37, 487–501 (2016).
Patel, A. et al. Differential RET signaling pathways drive development of the enteric lymphoid and nervous systems. Sci. Signal. 5, ra55 (2012).
Frykman, P. K., Cheng, Z., Wang, X. & Dhall, D. Enterocolitis causes profound lymphoid depletion in endothelin receptor B- and endothelin 3-null mouse models of Hirschsprung-associated enterocolitis. Eur. J. Immunol. 45, 807–817 (2015).
Di Giovangiulio, M. et al. The neuromodulation of the intestinal immune system and its relevance in inflammatory bowel disease. Front. Immunol. 6, 590 (2015).
Sofroniew, M. V. et al. Genetically-targeted and conditionally-regulated ablation of astroglial cells in the central, enteric and peripheral nervous systems in adult transgenic mice. Brain Res. 835, 91–95 (1999).
Burns, A. J. et al. White paper on guidelines concerning enteric nervous system stem cell therapy for enteric neuropathies. Dev. Biol. 417, 229–251 (2016).
Burns, A. J. & Thapar, N. Neural stem cell therapies for enteric nervous system disorders. Nat. Rev. Gastroenterol. Hepatol. 11, 317–328 (2014).
Workman, M. J. et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat. Med. 23, 49–59 (2016).
Goto, K. et al. Activation of 5-HT4 receptors facilitates neurogenesis from transplanted neural stem cells in the anastomotic ileum. J. Physiol. Sci. 66, 67–76 (2016).
Gershon, M. D. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 20, 14–21 (2013).
Wood, J. D. Enteric nervous system neuropathy: repair and restoration. Curr. Opin. Gastroenterol. 27, 106–111 (2011).
Takenouchi, T. et al. Hirschsprung disease as a yet undescribed phenotype in a patient with ARID1B mutation. Am. J. Med. Genet. A 170A, 3249–3252 (2016).
Zhu, J. J., Kam, M. K., Garcia-Barceló, M. M., Tam, P. K. & Lui, V. C. HOXB5 binds to multi-species conserved sequence (MCS+9.7) of RET gene and regulates RET expression. Int. J. Biochem. Cell Biol. 51, 142–149 (2014).
Acknowledgements
R.O.H. is supported by the Burroughs Wellcome Fund Clinical Scientist Award in Translational Research (1008525), the Children's Discovery Institute at Washington University School of Medicine (MD-11-2013-269), FDA (R01FD005133), Penn Medicine Neuroscience Center, March of Dimes 6-FY15-235, PennCHOP Microbiome Program (FP00021900), NIH Common Fund (1OT2OD023859-01), Canadian Institutes of Health Research (201610PJT), and by generous support from the Irma and Norman Braman Endowment, The Suzi and Scott Lustgarten Center Endowment and The Children's Hospital of Philadelphia Research Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Heuckeroth, R. Hirschsprung disease — integrating basic science and clinical medicine to improve outcomes. Nat Rev Gastroenterol Hepatol 15, 152–167 (2018). https://doi.org/10.1038/nrgastro.2017.149
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2017.149
This article is cited by
-
A branching model of lineage differentiation underpinning the neurogenic potential of enteric glia
Nature Communications (2023)
-
Hirschsprung disease
Nature Reviews Disease Primers (2023)
-
Downregulation of miR-144 blocked the proliferation and invasion of nerve cells in Hirschsprung disease by regulating Transcription Factor AP 4 (TFAP4)
Pediatric Surgery International (2023)
-
Suppression of PGE2/EP2 signaling alleviates Hirschsprung disease by upregulating p38 mitogen-activated protein kinase activity
Journal of Molecular Medicine (2023)
-
Abnormally elevated expression of ACTA2 of circular smooth muscle leads to hyperactive contraction in aganglionic segments of HSCR
Pediatric Surgery International (2023)