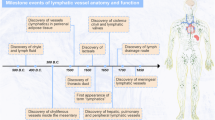
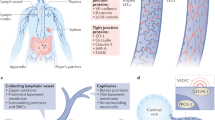
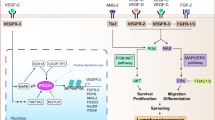
Key Points
-
Intestinal lymphatic vessels are critical for fat absorption and gut immunosurveillance
-
Some of the molecular mechanisms of intestinal lymphatic development and maintenance are distinct from other lymphatic vessel beds
-
Intestinal lymphatic dysfunction is likely a contributing factor in intestinal pathologies such as IBD
-
Modulating intestinal lymphatic patterning and/or function might provide novel therapies for gastrointestinal tract diseases
Abstract
The mammalian intestine is richly supplied with lymphatic vasculature, which has functions ranging from maintenance of interstitial fluid balance to transport of antigens, antigen-presenting cells, dietary lipids and fat-soluble vitamins. In this Review, we provide in-depth information concerning the organization and structure of intestinal lymphatics, the current view of their developmental origins, as well as molecular mechanisms of intestinal lymphatic patterning and maintenance. We will also discuss physiological aspects of intestinal lymph flow regulation and the known and emerging roles of intestinal lymphatic vessels in human diseases, such as IBD, infection and cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Trapnell, D. H. Man's understanding of the lymphatics, with particular reference to the lung. J. R. Soc. Med. 58, 37–40 (1965).
Chikly, B. Who discovered the lymphatic system? Lymphology 30, 186–193 (1997).
Asseli, G. De lactibus sive lacteis venis, quarto vasorum mesaraicorum genere, novo invento, dissertatio [Latin] (Henric-Petrinis, 1627).
Mayer, E. A., Tillisch, K. & Gupta, A. Gut/brain axis and the microbiota. J. Clin. Invest. 125, 926–938 (2015).
Belkaid, Y. & Hand, T. W. Role of the microbiota in immunity and inflammation. Cell 157, 121–141 (2014).
Powell, D. W., Pinchuk, I. V., Saada, J. I., Chen, X. & Mifflin, R. C. Mesenchymal cells of the intestinal lamina propria. Annu. Rev. Physiol. 73, 213–237 (2011).
Yang, Y. et al. Anti-VEGF- and anti-VEGF receptor-induced vascular alteration in mouse healthy tissues. Proc. Natl Acad. Sci. USA 110, 12018–12023 (2013).
Kamba, T. et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am. J. Physiol. Heart Circ. Physiol. 290, H560–H576 (2006).
Mowat, A. M. & Agace, W. W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 14, 667–685 (2014).
Garrett, W. S., Gordon, J. I. & Glimcher, L. H. Homeostasis and inflammation in the intestine. Cell 140, 859–870 (2010).
van der Flier, L. G. & Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 71, 241–260 (2009).
Eberl, G. & Lochner, M. The development of intestinal lymphoid tissues at the interface of self and microbiota. Mucosal Immunol. 2, 478–485 (2009).
Baluk, P. et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 204, 2349–2362 (2007).
Schulte-Merker, S., Sabine, A. & Petrova, T. V. Lymphatic vascular morphogenesis in development, physiology, and disease. J. Cell Biol. 193, 607–618 (2011).
Scallan, J. P., Zawieja, S. D., Castorena-Gonzalez, J. A. & Davis, M. J. Lymphatic pumping: mechanics, mechanisms and malfunction. J. Physiol. 594, 5749–5768 (2016).
Jang, J. Y. et al. Conditional ablation of LYVE-1+cells unveils defensive roles of lymphatic vessels in intestine and lymph nodes. Blood 122, 2151–2161 (2013).
Unthank, J. L. & Bohlen, H. G. Lymphatic pathways and role of valves in lymph propulsion from small-intestine. Am. J. Physiol. 254, G389–G398 (1988).
Norrmén, C. et al. Liprin beta 1 is highly expressed in lymphatic vasculature and is important for lymphatic vessel integrity. Blood 115, 906–909 (2010).
Karkkainen, M. J. et al. A model for gene therapy of human hereditary lymphedema. Proc. Natl Acad. Sci. USA 98, 12677–12682 (2001).
Farstad, I., Malavasi, F., Haraldsen, G., Huitfeldt, H. & Brandtzaeg, P. CD38 is a marker of human lacteals. Virchows Arch. 441, 605–613 (2002).
Bernier-Latmani, J. et al. DLL4 promotes continuous adult intestinal lacteal regeneration and dietary fat transport. J. Clin. Invest. 125, 4572–4586 (2015).
Papp, M., Röhlich, P., Rusznyák, I. & Törö, I. An electron microscopic study of the central lacteal in the intestinal villus of the cat. Z. Zellforsch. Mikrosk. Anat. 57, 475–486 (1962).
Ohtani, O. & Ohtani, Y. Organization and developmental aspects of lymphatic vessels. Arch. Histol. Cytol. 71, 1–22 (2008).
Choe, K. et al. Intravital imaging of intestinal lacteals unveils lipid drainage through contractility. J. Clin. Invest. 125, 4042–4052 (2015).
Bernier-Latmani, J. & Petrova, T. V. High-resolution 3D analysis of mouse small-intestinal stroma. Nat. Protoc. 11, 1617–1629 (2016).
Nurmi, H. et al. VEGF-C is required for intestinal lymphatic vessel maintenance and lipid absorption. EMBO Mol. Med. 7, 1418–1425 (2015).
Wernstedt Asterholm, I. et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 20, 103–118 (2014).
Ivanov, S. et al. CCR7 and IRF4-dependent dendritic cells regulate lymphatic collecting vessel permeability. J. Clin. Invest. 126, 1581–1591 (2016).
Rafii, S., Butler, J. M. & Ding, B.-S. Angiocrine functions of organ-specific endothelial cells. Nature 529, 316–325 (2016).
Hatch, J. & Mukouyama, Y. S. Spatiotemporal mapping of vascularization and innervation in the fetal murine intestine. Dev. Dyn. 244, 56–68 (2015).
Kim, K. E., Sung, H.-K. & Koh, G. Y. Lymphatic development in mouse small intestine. Dev. Dyn. 236, 2020–2025 (2007).
Stanczuk, L. et al. cKit lineage hemogenic endothelium-derived cells contribute to mesenteric lymphatic vessels. Cell Rep. 10, 1708–1721 (2015).
Mahadevan, A. et al. The left-right Pitx2 pathway drives organ-specific arterial and lymphatic development in the intestine. Dev. Cell 31, 690–706 (2014).
Wells, J. M. & Spence, J. R. How to make an intestine. Development 141, 752–760 (2014).
Norrmén, C. et al. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J. Cell Biol. 185, 439–457 (2009).
Makinen, T. et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat. Med. 7, 199–205 (2001).
Tammela, T. et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 454, 656–660 (2008).
Zarkada, G., Heinolainen, K., Makinen, T., Kubota, Y. & Alitalo, K. VEGFR3 does not sustain retinal angiogenesis without VEGFR2. Proc. Natl Acad. Sci. USA 112, 761–766 (2015).
Karkkainen, M. J. et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 5, 74–80 (2004).
Xu, Y. L. et al. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J. Cell Biol. 188, 115–130 (2010).
Haiko, P. et al. Deletion of vascular endothelial growth factor c (Vegf-c) and Vegf-d is not equivalent to Vegf receptor 3 deletion in mouse embryos. Mol. Cell. Biol. 28, 4843–4850 (2008).
Lapinski, P. E. et al. RASA1 maintains the lymphatic vasculature in a quiescent functional state in mice. J. Clin. Invest. 122, 733–747 (2012).
Saharinen, P. et al. Claudin-like protein 24 interacts with the VEGFR-2 and VEGFR-3 pathways and regulates lymphatic vessel development. Genes Dev. 24, 875–880 (2010).
Liu, X. et al. Temporal and spatial regulation of Epsin abundance and VEGFR3 signaling are required for lymphatic valve formation and function. Sci. Signal. 7, ra97 (2014).
Gupta, S. et al. Binding of Ras to phosphoinositide 3-kinase p110 alpha is required for Ras-driven tumorigenesis in mice. Cell 129, 957–968 (2007).
Mouta-Bellum, C. et al. Organ-specific lymphangiectasia, arrested lymphatic sprouting, and maturation defects resulting from gene-targeting of the PI3K regulatory isoforms p85 alpha, p55 alpha, and p50 alpha. Dev. Dyn. 238, 2670–2679 (2009).
Wigle, J. T. & Oliver, G. Prox1 function is required for the development of the murine lymphatic system. Cell 98, 769–778 (1999).
Harvey, N. L. et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat. Genet. 37, 1072–1081 (2005).
Escobedo, N. et al. Restoration of lymphatic function rescues obesity in Prox1-haploinsufficient mice. JCI Insight 1, e85096 (2016).
Chen, L. et al. Tbx1 regulates Vegfr3 and is required for lymphatic vessel development. J. Cell Biol. 189, 417–424 (2010).
Augustin, H. G., Koh, G. Y., Thurston, G. & Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 10, 165–177 (2009).
Gale, N. W. et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev. Cell 3, 411–423 (2002).
Shimoda, H. et al. Abnormal recruitment of periendothelial cells to lymphatic capillaries in digestive organs of angiopoietin-2-deficient mice. Cell Tissue Res. 328, 329–337 (2007).
Shen, B. et al. Genetic dissection of Tie pathway in mouse lymphatic maturation and valve development. Arterioscler. Thromb. Vasc. Biol. 34, 1221–1230 (2014).
Qu, X., Zhou, B. & Baldwin, H. S. Tie1 is required for lymphatic valve and collecting vessel development. Dev. Biol. 399, 117–128 (2015).
Zheng, W. et al. Angiopoietin 2 regulates the transformation and integrity of lymphatic endothelial cell junctions. Genes Dev. 28, 1592–1603 (2014).
Hoopes, S. L., Willcockson, H. H. & Caron, K. M. Characteristics of multi-organ lymphangiectasia resulting from temporal deletion of calcitonin receptor-like receptor in adult mice. PLoS ONE 7, e45261 (2012).
Dijk, W. & Kersten, S. Regulation of lipid metabolism by angiopoietin-like proteins. Curr. Opin. Lipidol. 27, 249–256 (2016).
Bäckhed, F., Crawford, P. A., O'Donnell, D. & Gordon, J. I. Postnatal lymphatic partitioning from the blood vasculature in the small intestine requires fasting-induced adipose factor. Proc. Natl Acad. Sci. USA 104, 606–611 (2007).
Lichtenstein, L. et al. Angptl4 protects against severe pro-inflammatory effects of dietary saturated fat by inhibiting lipoprotein lipase-dependent uptake of fatty acids in mesenteric lymph node macrophages. Cell Metab. 12, 580–592 (2010).
Bertozzi, C. C. et al. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood 116, 661–670 (2010).
Uhrin, P. et al. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood 115, 3997–4005 (2010).
Abtahian, F. et al. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science 299, 247–251 (2003).
Suzuki-Inoue, K. et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood 107, 542–549 (2006).
Hess, P. R. et al. Platelets mediate lymphovenous hemostasis to maintain blood-lymphatic separation throughout life. J. Clin. Invest. 124, 273–284 (2014).
Suzuki-Inoue, K. et al. Essential in vivo roles of the C-type lectin receptor Clec-2: embryonic/neonatal lethality of Clec-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of Clec-2-deficient platelets. J. Biol. Chem. 285, 24494–24507 (2010).
Welsh, J. D., Kahn, M. L. & Sweet, D. T. Lymphovenous hemostasis and the role of platelets in regulating lymphatic flow and lymphatic vessel maturation. Blood 128, 1169–1173 (2016).
Bohmer, R. et al. Regulation of developmental lymphangiogenesis by Syk+ leukocytes. Dev. Cell 18, 437–449 (2010).
Sabine, A. et al. FOXC2 and fluid shear stress stabilize postnatal lymphatic vasculature. J. Clin. Invest. 125, 3861–3877 (2015).
Makinen, T. et al. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 19, 397–410 (2005).
Levet, S. et al. Bone morphogenetic protein 9 (BMP9) controls lymphatic vessel maturation and valve formation. Blood 122, 598–607 (2013).
Sabine, A. et al. Mechanotransduction, PROX1, and FOXC2 cooperate to control Connexin37 and Calcineurin during lymphatic-valve formation. Dev. Cell 22, 430–445 (2012).
Scallan, J. P., Davis, M. J. & Huxley, V. H. Permeability and contractile responses of collecting lymphatic vessels elicited by atrial and brain natriuretic peptides. J. Physiol. 591, 5071–5081 (2013).
Armulik, A., Genové, G. & Betsholtz, C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 21, 193–215 (2011).
Mansbach, C. M. & Siddiqi, S. A. The biogenesis of chylomicrons. Annu. Rev. Physiol. 72, 315–333 (2010).
Dash, S., Xiao, C., Morgantini, C. & Lewis, G. F. New insights into the regulation of chylomicron production. Annu. Rev. Nutr. 35, 265–294 (2015).
Karupaiah, T. & Sundram, K. Effects of stereospecific positioning of fatty acids in triacylglycerol structures in native and randomized fats: a review of their nutritional implications. Nutr. Metab. 4, 16–16 (2007).
Lo, C.-M. et al. Why does the gut choose apolipoprotein B48 but not B100 for chylomicron formation? Am. J. Physiol. Gastrointest. Liver Physiol. 294, G344–G352 (2008).
Iqbal, J. & Hussain, M. M. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 296, E1183–E1194 (2009).
Hayashi, H. et al. Fat feeding increases size, but not number, of chylomicrons produced by small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 259, G709–G719 (1990).
Clementi, F. & Palade, G. E. Intestinal capillaries: I. Permeability to peroxidase and ferritin. J. Cell Biol. 41, 33–58 (1969).
Altmann, S. W. et al. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science 303, 1201–1204 (2004).
Brunham, L. R. et al. β-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat. Med. 13, 340–347 (2007).
Berge, K. E. et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 290, 1771–1775 (2000).
Lee, M.-H. et al. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat. Genet. 27, 79–83 (2001).
Zhang, L. S. et al. ABCG5/G8 deficiency in mice reduces dietary triacylglycerol and cholesterol transport into the lymph. Lipids 50, 371–379 (2015).
Wang, X. & Rader, D. J. Molecular regulation of macrophage reverse cholesterol transport. Curr. Opin. Cardiol. 22, 368–372 (2007).
Lim, H. Y. et al. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-mediated transport of HDL. Cell Metab. 17, 671–684 (2013).
Martel, C. et al. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J. Clin. Invest. 123, 1571–1579 (2013).
Bura, K. S. et al. Intestinal SR-BI does not impact cholesterol absorption or transintestinal cholesterol efflux in mice. J. Lipid Res. 54, 1567–1577 (2013).
Mardones, P. et al. Hepatic cholesterol and bile acid metabolism and intestinal cholesterol absorption in scavenger receptor class B type I-deficient mice. J. Lipid Res. 42, 170–180 (2001).
Van Dyck, F. et al. Loss of the Plagl2 transcription factor affects lacteal uptake of chylomicrons. Cell Metab. 6, 406–413 (2007).
Eroglu, A. & Harrison, E. H. Carotenoid metabolism in mammals, including man: formation, occurrence, and function of apocarotenoids: thematic review series: fat-soluble vitamins: vitamin A. J. Lipid Res. 54, 1719–1730 (2013).
Traber, M. G. Mechanisms for the prevention of vitamin E excess. J. Lipid Res. 54, 2295–2306 (2013).
Dahan, A. & Hoffman, A. Evaluation of a chylomicron flow blocking approach to investigate the intestinal lymphatic transport of lipophilic drugs. Eur. J. Pharm. Sci. 24, 381–388 (2005).
Shearer, M. J. & Newman, P. Metabolism and cell biology of vitamin K. Thromb. Haemost. 100, 530–547 (2008).
Hornef, M. W., Frisan, T., Vandewalle, A., Normark, S. & Richter-Dahlfors, A. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J. Exp. Med. 195, 559–570 (2002).
Neal, M. D. et al. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J. Immunol. 176, 3070–3079 (2006).
McDole, J. R. et al. Goblet cells deliver luminal antigen to CD103+ DCs in the small intestine. Nature 483, 345–349 (2012).
Vreugdenhil, A. C. E. et al. Lipopolysaccharide (LPS)-binding protein mediates LPS detoxification by chylomicrons. J. Immunol. 170, 1399–1405 (2003).
Ghoshal, S., Witta, J., Zhong, J., de Villiers, W. & Eckhardt, E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 50, 90–97 (2009).
Drucker, D. J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 24, 15–30 (2016).
D'Alessio, D. et al. Fasting and postprandial concentrations of GLP-1 in intestinal lymph and portal plasma: evidence for selective release of GLP-1 in the lymph system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R2163–R2169 (2007).
Lu, W. J. et al. The regulation of the lymphatic secretion of glucagon-like peptide-1 (GLP-1) by intestinal absorption of fat and carbohydrate. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G963–G971 (2007).
Deacon, C. F., Johnsen, A. H. & Holst, J. J. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J. Clin. Endocrinol. Metab. 80, 952–957 (1995).
Holst, J. J. The physiology of glucagon-like peptide 1. Physiol. Rev. 87, 1409–1439 (2007).
Palay, S. L. & Karlin, L. J. An electron microscopic study of the intestinal villus: II. The pathway of fat absorption. J. Biophys. Biochem. Cytol. 5, 373–384 (1959).
Casley-Smith, J. R. Identification of chylomicra and lipoproteins in tissue sections and their passage into jejunal lacteals. J. Cell Biol. 15, 259–277 (1962).
Tso, P. & Balint, J. A. Formation and transport of chylomicrons by enterocytes to the lymphatics. Am. J. Physiol. 250, G715–G726 (1986).
Sabesin, S. M. & Frase, S. Electron-microscopic studies of assembly, intracellular-transport, and secretion of chylomicrons by rat intestine. J. Lipid Res. 18, 496–511 (1977).
Reed, A. L., Rowson, S. A. & Dixon, J. B. Demonstration of ATP dependent, transcellular transport of lipid across the lymphatic endothelium using an in vitro model of the lacteal. Pharm. Res. 30, 3271–3280 (2013).
Ashworth, C., Stembridge, V. & Sanders, E. Lipid absorption, transport and hepatic assimilation studied with electron microscopy. Am. J. Physiol. 198, 1326 (1960).
Ottaviani, G. & Azzali, G. Ultrastructure of lymphatic vessels in some functional conditions. Acta Anat. Suppl. (Basel) 73 (Suppl. 56), 325–336 (1969).
Dobbins, W. & Rollins, E. Intestinal mucosal lymphatic permeability: an electron microscopic study of endothelial vesicles and cell junctions. J. Ultrastruct. Res. 33, 29–59 (1970).
Dobbins, W. O. Intestinal mucosal lacteal in transport of macromolecules and chylomicrons. Am. J. Clin. Nutr. 24, 77–90 (1971).
Listenberger, L. L. et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl Acad. Sci. USA 100, 3077–3082 (2003).
Borradaile, N. M. et al. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J. Lipid Res. 47, 2726–2737 (2006).
Gayer, C. P. & Basson, M. D. The effects of mechanical forces on intestinal physiology and pathology. Cell. Signal. 21, 1237–1244 (2009).
Güldner, F.-H., Wolff, J. R. & Keyserlingk, D. G. Fibroblasts as a part of the contractile system in duodenal villi of rat. Z. Zellforsch. Mikrosk. Anat. 135, 349–360 (1972).
Lee, J. S. Contraction of villi and fluid transport in dog jejunal mucosa in-vitro. Am. J. Physiol. 221, 488–495 (1971).
Womack, W. A. et al. Quantitative assessment of villous motility. Am. J. Physiol. 252, G250–G256 (1987).
Tso, P., Pitts, V. & Granger, D. N. Role of lymph-flow in intestinal chylomicron transport. Am. J. Physiol. 249, G21–G28 (1985).
Zawieja, D. C. Contractile physiology of lymphatics. Lymphat. Res. Biol. 7, 87–96 (2009).
Fu, Y.-Y., Peng, S.-J., Lin, H.-Y., Pasricha, P. J. & Tang, S.-C. 3D imaging and illustration of mouse intestinal neurovascular complex. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G1–G11 (2013).
Ichikawa, S., Kasahara, D., Iwanaga, T., Uchino, S. & Fujita, T. Peptidergic nerve-terminals associated with the central lacteal lymphatics in the ileal villi of dogs. Arch. Histol. Cytol. 54, 311–320 (1991).
Ichikawa, S., Kyoda, K., Iwanaga, T., Fujita, T. & Uchino, S. Nerve-terminals associated with the central lacteal lymphatics in the duodenal and ileal villi of the monkey. Acta Anat. (Basel) 146, 14–21 (1993).
Poole, D. P. et al. Feeding-dependent activation of enteric cells and sensory neurons by lymphatic fluid: evidence for a neurolymphocrine system. Am. J. Physiol. Gastrointest. Liver Physiol. 306, G686–G698 (2014).
Mignini, F., Sabbatini, M., Coppola, L. & Cavallotti, C. Analysis of nerve supply pattern in human lymphatic vessels of young and old men. Lymphat. Res. Biol. 10, 189–197 (2012).
Sacchi, G., Weber, E., Agliano, M. & Comparini, L. Subendothelial nerve-fibers in bovine mesenteric lymphatics — an ultrastructural and immunohistochemical study. Lymphology 27, 90–96 (1994).
Alessandrini, C. et al. Cholinergic and adrenergic-innervation of mesenterial lymph vessels in guinea-pig. Lymphology 14, 1–6 (1981).
Pabst, O. & Mowat, A. M. Oral tolerance to food protein. Mucosal Immunol. 5, 232–239 (2012).
Cerovic, V., Bain, C. C., Mowat, A. M. & Milling, S. W. F. Intestinal macrophages and dendritic cells: what's the difference? Trends Immunol. 35, 270–277 (2014).
Schulz, O. et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J. Exp. Med. 206, 3101–3114 (2009).
Bogunovic, M. et al. Origin of the lamina propria dendritic cell network. Immunity 31, 513–525 (2009).
Cerovic, V. et al. Intestinal CD103- dendritic cells migrate in lymph and prime effector T cells. Mucosal Immunol. 6, 104–113 (2013).
Chang, S.-Y. et al. Circulatory antigen processing by mucosal dendritic cells controls CD8+ T cell activation. Immunity 38, 153–165 (2013).
Farache, J. et al. Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity 38, 581–595 (2013).
Förster, R., Braun, A. & Worbs, T. Lymph node homing of T cells and dendritic cells via afferent lymphatics. Trends Immunol. 33, 271–280 (2012).
Ohl, L. et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 21, 279–288 (2004).
Betterman, K. L. & Harvey, N. L. The lymphatic vasculature: development and role in shaping immunity. Immunol. Rev. 271, 276–292 (2016).
Lammermann, T. et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453, 51–55 (2008).
Worbs, T. et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J. Exp. Med. 203, 519–527 (2006).
Johansson-Lindbom, B. et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 202, 1063–1073 (2005).
Sonnenberg, G. F. & Artis, D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat. Med. 21, 698–708 (2015).
Cella, M. et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725 (2009).
Hepworth, M. R. et al. Innate lymphoid cells regulate CD4+ T cell responses to intestinal commensal bacteria. Nature 498, 113–117 (2013).
Sawa, S. et al. ROR gamma t+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat. Immunol. 12, 320–326 (2011).
Hanash, A. M. et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity 37, 339–350 (2012).
Aparicio-Domingo, P. et al. Type 3 innate lymphoid cells maintain intestinal epithelial stem cells after tissue damage. J. Exp. Med. 212, 1783–1791 (2015).
Mackley, E. C. et al. CCR7-dependent trafficking of RORγ+ ILCs creates a unique microenvironment within mucosal draining lymph nodes. Nat. Commun. 6, 5862 (2015).
Abadie, V. et al. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood 106, 1843–1850 (2005).
Hampton, H. R., Bailey, J., Tomura, M., Brink, R. & Chtanova, T. Microbe-dependent lymphatic migration of neutrophils modulates lymphocyte proliferation in lymph nodes. Nat. Commun. 6, 7139 (2015).
Tomura, M. et al. Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice. J. Clin. Invest. 120, 883–893 (2010).
Umar, S. B. & DiBaise, J. K. Protein-losing enteropathy: case illustrations and clinical review. Am. J. Gastroenterol. 105, 43–49 (2010).
Freeman, H. J. & Nimmo, M. Intestinal lymphangiectasia in adults. World. J. Gastrointest. Oncol. 3, 19–23 (2011).
Ingle, S. B. & Hinge, C. R. Primary intestinal lymphangiectasia: minireview. World J. Clin. Cases 2, 528–533 (2014).
Sawane, M. et al. Apelin inhibits diet-induced obesity by enhancing lymphatic and blood vessel integrity. Diabetes 62, 1970–1980 (2013).
Brouillard, P., Boon, L. & Vikkula, M. Genetics of lymphatic anomalies. J. Clin. Invest. 124, 898–904 (2014).
Alders, M. et al. Mutations in CCBE1 cause generalized lymph vessel dysplasia in humans. Nat. Genet. 41, 1272–1274 (2009).
Alders, M. et al. Hennekam syndrome can be caused by FAT4 mutations and be allelic to Van Maldergem syndrome. Hum. Genet. 133, 1161–1167 (2014).
Hennekam, R. C. M. et al. Autosomal recessive intestinal lymphangiectasia and lymphedema, with facial anomalies and mental retardation. Am. J. Med. Genet. 34, 593–600 (1989).
Jeltsch, M. et al. CCBE1 enhances lymphangiogenesis via a disintegrin and metalloprotease with thrombospondin motifs-3-mediated vascular endothelial growth factor-C activation. Circulation 129, 1962–1971 (2014).
Le Guen, L. et al. Ccbe1 regulates Vegfc-mediated induction of Vegfr3 signaling during embryonic lymphangiogenesis. Development 141, 1239–1249 (2014).
Saburi, S. et al. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat. Genet. 40, 1010–1015 (2008).
Karkkainen, M. J. et al. Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat. Genet. 25, 153–159 (2000).
Irrthum, A., Karkkainen, M. J., Devriendt, K., Alitalo, K. & Vikkula, M. Congenital hereditary lymphedema caused by a mutation that inactivates VEGFR3 tyrosine kinase. Am. J. Hum. Genet. 67, 295–301 (2000).
Ghalamkarpour, A. et al. Hereditary lymphedema type I associated with VEGFR3 mutation: the first de novo case and atypical presentations. Clin. Genet. 70, 330–335 (2006).
Ghalamkarpour, A. et al. Recessive primary congenital lymphoedema caused by a VEGFR3 mutation. J. Med. Genet. 46, 399–404 (2009).
Gordon, K. et al. Mutation in vascular endothelial growth factor-C, a ligand for vascular endothelial growth factor receptor-3, is associated with autosomal dominant Milroy-like primary lymphedema. Circ. Res. 112, 956–960 (2013).
Balboa-Beltran, E. et al. A novel stop mutation in the vascular endothelial growth factor-C gene (VEGFC) results in Milroy-like disease. J. Med. Genet. 51, 475–478 (2014).
Fotiou, E. et al. Novel mutations in PIEZO1 cause an autosomal recessive generalized lymphatic dysplasia with non-immune hydrops fetalis. Nat. Commun. 6, 8085 (2015).
Lukacs, V. et al. Impaired PIEZO1 function in patients with a novel autosomal recessive congenital lymphatic dysplasia. Nat. Commun. 6, 8329 (2015).
Li, J. et al. Piezo1 integration of vascular architecture with physiological force. Nature 515, 279–282 (2014).
Calabrese, C., Pironi, L. & Di Febo, G. Capsule endoscopy revealing small-intestinal lymphangiectasia and GI stromal tumor polyps in neurofibromatosis type 1. Gastrointest. Endosc. 64, 130–131 (2006).
Desai, A. P., Guvenc, B. H. & Carachi, R. Evidence for medium chain triglycerides in the treatment of primary intestinal lymphangiectasia. Eur. J. Pediatr. Surg. 19, 241–245 (2009).
Vignes, S. & Bellanger, J. Primary intestinal lymphangiectasia (Waldmann's disease). Orphanet J. Rare Dis. 3, 5–5 (2008).
Wen, J., Tang, Q., Wu, J., Wang, Y. & Cai, W. Primary intestinal lymphangiectasia: four case reports and a review of the literature. Dig. Dis. Sci. 55, 3466–3472 (2010).
de Souza, H. S. P. & Fiocchi, C. Immunopathogenesis of IBD: current state of the art. Nat. Rev. Gastroenterol. Hepatol. 13, 13–27 (2016).
Van Kruiningen, H. J. & Colombel, J.-F. The forgotten role of lymphangitis in Crohn's disease. Gut 57, 1–4 (2008).
von der Weid, P.-Y., Rehal, S. & Ferraz, J. G. Role of the lymphatic system in the pathogenesis of Crohn's disease. Curr. Opin. Gastroenterol. 27, 335–341 (2011).
von der Weid, P. Y. & Rainey, K. J. Lymphatic system and associated adipose tissue in the development of inflammatory bowel disease. Aliment. Pharmacol. Ther. 32, 697–711 (2010).
Franke, A. et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat. Genet. 42, 1118–1125 (2010).
Barrett, J. C. et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat. Genet. 40, 955–962 (2008).
Haberman, Y. et al. Pediatric Crohn disease patients exhibit specific ileal transcriptome and microbiome signature. J. Clin. Invest. 124, 3617–3633 (2014).
Ganta, V. C. et al. Angiopoietin-2 in experimental colitis. Inflamm. Bowel Dis. 16, 1029–1039 (2010).
D'Alessio, S. et al. VEGF-C-dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J. Clin. Invest. 124, 3863–3878 (2014).
Jurisic, G., Sundberg, J. P. & Detmar, M. Blockade of VEGF receptor-3 aggravates inflammatory bowel disease and lymphatic vessel enlargement. Inflamm. Bowel Dis. 19, 1983–1989 (2013).
Vetrano, S. et al. The lymphatic system controls intestinal inflammation and inflammation-associated colon cancer through the chemokine decoy receptor D6. Gut 59, 197–206 (2010).
Yao, L. C., Baluk, P., Srinivasan, R. S., Oliver, G. & McDonald, D. M. Plasticity of button-like junctions in the endothelium of airway lymphatics in development and inflammation. Am. J. Pathol. 180, 2561–2575 (2012).
Alexander, J. S., Chaitanya, G. V., Grisham, M. B. & Boktor, M. Emerging roles of lymphatics in inflammatory bowel disease. Ann. NY Acad. Sci. 1207, E75–E85 (2010).
D'Alessio, S., Tacconi, C., Fiocchi, C. & Danese, S. Advances in therapeutic interventions targeting the vascular and lymphatic endothelium in inflammatory bowel disease. Curr. Opin. Gastroenterol. 29, 608–613 (2013).
Becker, F. et al. Downregulation of Foxc2 increased susceptibility to experimental colitis: influence of lymphatic drainage function? Inflamm. Bowel Dis. 21, 1282–1296 (2015).
von der Weid, P.-Y. & Rehal, S. Lymphatic pump function in the inflamed gut. Ann. NY Acad. Sci. 1207, E69–E74 (2010).
von der Weid, P.-Y. & Muthuchamy, M. Regulatory mechanisms in lymphatic vessel contraction under normal and inflammatory conditions. Pathophysiology 17, 263–276 (2010).
Chakraborty, S., Davis, M. J. & Muthuchamy, M. Emerging trends in the pathophysiology of lymphatic contractile function. Semin. Cell Dev. Biol. 38, 55–66 (2015).
Mathias, R. & von der Weid, P.-Y. Involvement of the NO-cGMP-KATP channel pathway in the mesenteric lymphatic pump dysfunction observed in the guinea pig model of TNBS-induced ileitis. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G623–G634 (2013).
Lynskey, N. N. et al. Rapid lymphatic dissemination of encapsulated group a Streptococci via lymphatic vessel endothelial receptor-1 interaction. PLoS Pathog. 11, e1005137 (2015).
Gonzalez, R. J., Lane, M. C., Wagner, N. J., Weening, E. H. & Miller, V. L. Dissemination of a highly virulent pathogen: tracking the early events that define infection. PLoS Pathog. 11, e1004587 (2015).
Iannacone, M. et al. Subcapsular sinus macrophages prevent CNS invasion upon peripheral infection with a neurotropic virus. Nature 465, 1079–1083 (2010).
Lerner, T. R. et al. Lymphatic endothelial cells are a replicative niche for Mycobacterium tuberculosis. J. Clin. Invest. 126, 1093–1108 (2016).
Gavrilovskaya, I. N., Gorbunova, E. E. & Mackow, E. R. Pathogenic hantaviruses direct the adherence of quiescent platelets to infected endothelial cells. J. Virol. 84, 4832–4839 (2010).
Liu, L. et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379, 2151–2161 (2012).
Perez-Lopez, A., Behnsen, J., Nuccio, S. P. & Raffatellu, M. Mucosal immunity to pathogenic intestinal bacteria. Nat. Rev. Immunol. 16, 135–148 (2016).
Morais da Fonseca, D. et al. Microbiota-dependent sequelae of acute infection compromise tissue-specific immunity. Cell 163, 354–366 (2015).
Peyrin-Biroulet, L. et al. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn's disease. Gut 61, 78–85 (2012).
Sartor, R. B. Microbial influences in inflammatory bowel diseases. Gastroenterology 134, 577–594 (2008).
Collins, S. M., Chang, C. & Mearin, F. Postinfectious chronic gut dysfunction: from bench to bedside. Am. J. Gastroenterol. Suppl. 1, 2–8 (2012).
Pan, S. Y. & Morrison, H. Epidemiology of cancer of the small intestine. World J. Gastrointest. Oncol. 3, 33–42 (2011).
Ferlay, J. et al. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase No.11. International Agency for Research on Cancer http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx?cancer=colorectal (2013).
Cunningham, D. et al. Colorectal cancer. Lancet 375, 1030–1047 (2010).
Baxter, N. N. et al. Lymph node evaluation in colorectal cancer patients: a population-based study. J. Natl Cancer Inst. 97, 219–225 (2005).
Mescoli, C. et al. Isolated tumor cells in regional lymph nodes as relapse predictors in stage I and II colorectal cancer. J. Clin. Oncol. 30, 965–971 (2012).
Rahbari, N. N. et al. Molecular detection of tumor cells in regional lymph nodes is associated with disease recurrence and poor survival in node-negative colorectal cancer: a systematic review and meta-analysis. J. Clin. Oncol. 30, 60–70 (2012).
Akagi, K. et al. Vascular endothelial growth factor-C (VEGF-C) expression in human colorectal cancer tissues. Br. J. Cancer 83, 887–891 (2000).
George, M. L. et al. VEGF-A, VEGF-C, and VEGF-D in colorectal cancer progression. Neoplasia 3, 420–427 (2001).
Royston, D. & Jackson, D. G. Mechanisms of lymphatic metastasis in human colorectal adenocarcinoma. J. Pathol. 217, 608–619 (2009).
Mlecnik, B. et al. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci. Transl Med. 8, 327ra26 (2016).
Bui, H. M. et al. Proteolytic activation defines distinct lymphangiogenic mechanisms for VEGFC and VEGFD. J. Clin. Invest. 126, 2167–2180 (2016).
He, Y. et al. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J. Natl Cancer Inst. 94, 819–825 (2002).
Tacconi, C. et al. Vascular endothelial growth factor c disrupts the endothelial lymphatic barrier to promote colorectal cancer invasion. Gastroenterology 148, 1438–1451.e8 (2015).
Kawakami, M., Yanai, Y., Hata, F. & Hirata, K. Vascular endothelial growth factor c promotes lymph node metastasis in a rectal cancer orthotopic model. Surg. Today 35, 131–138 (2005).
Guinney, J. et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 21, 1350–1356 (2015).
Dolcetti, R. et al. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am. J. Pathol. 154, 1805–1813 (1999).
Smyrk, T. C., Watson, P., Kaul, K. & Lynch, H. T. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer 91, 2417–2422 (2001).
Sato, T. et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Planas-Paz, L. et al. Mechanoinduction of lymph vessel expansion. EMBO J. 31, 788–804 (2012).
Karasov, W. H., Buddington, R. K. & Diamond, J. M. in Transport Processes, Iono- and Osmoregulation (eds Gilles, R. & Gilles-Baillien, M.) 227–239 (Springer, 1985).
Caviedes-Vidal, E. et al. The digestive adaptation of flying vertebrates: high intestinal paracellular absorption compensates for smaller guts. Proc. Natl Acad. Sci. USA 104, 19132–19137 (2007).
Wallace, K. N., Akhter, S., Smith, E. M., Lorent, K. & Pack, M. Intestinal growth and differentiation in zebrafish. Mech. Dev. 122, 157–173 (2005).
Lametschwandtner, A., Lametschwandtner, U., Radner, C. & Minnich, B. Spatial growth and pattern formation in the small intestine microvascular bed from larval to adult Xenopus laevis: a scanning electron microscope study of microvascular corrosion casts. Anat. Embryol. (Berl.) 211, 535–547 (2006).
Krause, W. J. Intestinal mucosa of the platypus, Ornithorhynchus anatinus. Anat. Rec. 181, 251–265 (1975).
Okuda, K. S. et al. lyve1 expression reveals novel lymphatic vessels and new mechanisms for lymphatic vessel development in zebrafish. Development 139, 2381–2391 (2012).
Reifel, C. W. & Travill, A. A. Structure and carbohydrate histochemistry of the intestine in 10 teleostean species. J. Morphol. 162, 343–359 (1979).
Noyan, A., Brot, N., Chaikoff, I. L. & Lossow, W. J. Pathway + form of absorption of palmitic acid in chicken. J. Lipid Res. 5, 538–541 (1964).
Fraser, R., Heslop, V. R., Murray, F. E. M. & Day, W. A. Ultrastructural studies of the portal transport of fat in chickens. Br. J. Exp. Pathol. 67, 783–791 (1986).
Harrop, C. & Hume, I. in Comparative Physiology: Primitive Mammals (eds Schmidt-Nielsen, K., Bolis, L. & Richard Taylor, C.) 63–78 (Cambridge Univ. Press, 2009).
Kawashima, Y., Sugimura, M., Hwang, Y. & Kudo, N. The lymph system in mice. Jpn. J. Vet. Res. 12, 69–78 (1964).
Carter, P. B. & Collins, F. M. The route of enteric infection in normal mice. J. Exp. Med. 139, 1189–1203 (1974).
Houston, S. A. et al. The lymph nodes draining the small intestine and colon are anatomically separate and immunologically distinct. Mucosal Immunol. 9, 468–478 (2016).
Escobedo, N. & Oliver, G. Lymphangiogenesis: origin, specification, and cell fate determination. Annu. Rev. Cell Dev. Biol. 32, 677–691 (2016).
Ulvmar, M. H. & Mäkinen, T. Heterogeneity in the lymphatic vascular system and its origin. Cardiovasc. Res. 111, 310–321 (2016).
Petrova, T. V. et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 21, 4593–4599 (2002).
Wigle, J. T. et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 21, 1505–1513 (2002).
Aspelund, A., Robciuc, M. R., Karaman, S., Makinen, T. & Alitalo, K. Lymphatic system in cardiovascular medicine. Circ. Res. 118, 515–530 (2016).
Bos, F. L. et al. CCBE1 is essential for mammalian lymphatic vascular development and enhances the lymphangiogenic effect of vascular endothelial growth factor-C in vivo. Circ. Res. 109, 486–491 (2011).
Janssen, L. et al. ADAMTS3 activity is mandatory for embryonic lymphangiogenesis and regulates placental angiogenesis. Angiogenesis 19, 53–65 (2016).
Sweet, D. T. et al. Lymph flow regulates collecting lymphatic vessel maturation in vivo. J. Clin. Invest. 125, 2995–3007 (2015).
Kanady, J. D., Dellinger, M. T., Munger, S. J., Witte, M. H. & Simon, A. M. Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax. Dev. Biol. 354, 253–266 (2011).
Kazenwadel, J. et al. GATA2 is required for lymphatic vessel valve development and maintenance. J. Clin. Invest. 125, 2979–2994 (2015).
Murtomaki, A. et al. Notch signaling functions in lymphatic valve formation. Development 141, 2446–2451 (2014).
Zhang, G. et al. EphB4 forward signalling regulates lymphatic valve development. Nat. Commun. 6, 6625 (2015).
Bazigou, E. et al. Genes regulating lymphangiogenesis control venous valve formation and maintenance in mice. J. Clin. Invest. 121, 2984–2992 (2011).
Tatin, F. et al. Planar cell polarity protein Celsr1 regulates endothelial adherens junctions and directed cell rearrangements during valve morphogenesis. Dev. Cell 26, 31–44 (2013).
Danussi, C. et al. EMILIN1/α9β1 integrin interaction is crucial in lymphatic valve formation and maintenance. Mol. Cell. Biol. 33, 4381–4394 (2013).
Bouvree, K. et al. Semaphorin3A, neuropilin-1, and plexinA1 are required for lymphatic valve formation. Circ. Res. 111, 437–445 (2012).
Jurisic, G. et al. An unexpected role of semaphorin3A-neuropilin-1 signaling in lymphatic vessel maturation and valve formation. Circ. Res. 111, 426–436 (2012).
Trevaskis, N. L., Kaminskas, L. M. & Porter, C. J. H. From sewer to saviour-targeting the lymphatic system to promote drug exposure and activity. Nat. Rev. Drug Discov. 14, 781–803 (2015).
Daggett, P. R., Wheeler, M. J. & Nabarro, J. D. N. Oral testosterone — reappraisal. Horm. Res. 9, 121–129 (1978).
Coert, A., Geelen, J., de Visser, J. & van der Vies, J. The pharmacology and metabolism of testosterone undecanoate (TU), a new orally active androgen. Acta Endocrinol. (Copenh.) 79, 789–800 (1975).
Horst, H. J. et al. Lymphatic absorption and metabolism of orally-administered testosterone undecanoate in man. Klin. Wochenschr. 54, 875–879 (1976).
Trevaskis, N. L., Shanker, R. M., Charman, W. N. & Porter, C. J. H. The mechanism of lymphatic access of two cholesteryl ester transfer protein inhibitors (CP524,515 and CP532,623) and evaluation of their impact on lymph lipoprotein profiles. Pharm. Res. 27, 1949–1964 (2010).
Lawless, E., Griffin, B. T., O'Mahony, A. & O'Driscoll, C. M. Exploring the impact of drug properties on the extent of intestinal lymphatic transport — in vitro and in vivo studies. Pharm. Res. 32, 1817–1829 (2015).
Khoo, S.-M., Shackleford, D. M., Porter, C. J. H., Edwards, G. A. & Charman, W. N. Intestinal lymphatic transport of halofantrine occurs after oral administration of a unit-dose lipid-based formulation to fasted dogs. Pharm. Res. 20, 1460–1465 (2003).
Han, S. et al. Targeted delivery of a model immunomodulator to the lymphatic system: comparison of alkyl ester versus triglyceride mimetic lipid prodrug strategies. J. Control. Release 177, 1–10 (2014).
Sugihara, J., Furuuchi, S., Nakano, K. & Harigaya, S. Studies on intestinal lymphatic absorption of drugs.1. Lymphatic absorption of alkyl ester derivatives and alpha-monoglyceride derivatives of drugs. J. Pharmacobiodyn. 11, 369–376 (1988).
Lu, Y. et al. Biomimetic reassembled chylomicrons as novel association model for the prediction of lymphatic transportation of highly lipophilic drugs via the oral route. Int. J. Pharm. 483, 69–76 (2015).
Hu, L. et al. Glyceride-mimetic prodrugs incorporating self-immolative spacers promote lymphatic transport, avoid first-pass metabolism, and enhance oral bioavailability. Angew. Chem. Int. Ed. 55, 13700–13705 (2016).
Herzog, D. B., Logan, R. & Kooistra, J. B. The Noonan syndrome with intestinal lymphangiectasia. J. Pediatr. 88, 270–272 (1976).
Roberts, A. E., Allanson, J. E., Tartaglia, M. & Gelb, B. D. Noonan syndrome. Lancet 381, 333–342 (2013).
Tatemichi, M., Nagata, H., Morinaga, S. & Kaneda, S. Protein-losing enteropathy caused by mesenteric vascular involvement of neurofibromatosis. Dig. Dis. Sci. 38, 1549–1553 (1993).
Atton, G. et al. The lymphatic phenotype in Turner syndrome: an evaluation of nineteen patients and literature review. Eur. J. Hum. Genet. 23, 1634–1639 (2015).
Acknowledgements
We apologize to many colleagues whose contributions were not discussed because of space limitations. We thank A. Sabine (University of Lausanne, Switzerland) for the confocal image of mesenteric lymphatics and both A. Sabine and D. Velin (Lausanne University Hospital, Switzerland) for critical reading of the manuscript. The work in the author's laboratory is supported by the Swiss National Science Foundation (CRSII3-141811, 31003A-156266 and CR32I3_166326), MEDIC, Gebert Rüf, Novartis and Swiss Bridge foundations, TheraLymph ERA-NET E-Rare Research Programme (FNS 31ER30_160674), The Commission for Technology and Innovation, Fondazione San Salvatore, Swiss Cancer League (KLS 3406-02-2014), and the People Programme (Marie Curie Actions) of the EU's Seventh Framework Programme FP7/2007 to 2013 under REA grant agreement 317250.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
T.V.P. received a research grant from Hoffman-La Roche to investigate angiogenesis inhibitors.
Supplementary information
Supplementary information S1 (table)
Intestinal lymphatic vascular defects in genetic mouse models (PDF 271 kb)
Glossary
- Lacteals
-
Central lymphatic capillaries in small intestinal villi.
- Button junctions
-
Discontinuous specialized cell–cell junctions found in normal mature lymphatic capillaries that facilitate the passage of interstitial components and immune cells into the lymphatic vessel lumen while maintaining vessel integrity
- Zipper junctions
-
Continuous and relatively impermeable junctions found in normal developing and mature lymphatic collecting vessels.
- Lymphatic valves
-
Intraluminal bi-leaflet structures, covered on both sides with endothelial cells; valves are present in lymphatic collecting vessels and they ensure unidirectional lymph flow towards blood circulation.
- Filopodia
-
Fine cytoplasmic, actin-rich cellular extensions usually observed in migrating cells, most often found in migrating blood or lymphatic endothelial cells.
- Chylous ascites
-
Accumulation of intestinal lymph in the abdomen, which appears white because of the presence of chylomicrons.
- Lymphangiectasia
-
Pathological dilation and malfunction of intestinal lymphatic vessels.
- Chylomicrons
-
Largest lipoprotein particle secreted by enterocytes after dietary long-chain fatty acid absorption; chylomicrons can also transport cholesterol, fat-soluble vitamins and lipopolysaccharide from the intestinal microbiota.
- Chyle
-
Fat-droplet rich, milky-white lymph draining from the intestine.
Rights and permissions
About this article
Cite this article
Bernier-Latmani, J., Petrova, T. Intestinal lymphatic vasculature: structure, mechanisms and functions. Nat Rev Gastroenterol Hepatol 14, 510–526 (2017). https://doi.org/10.1038/nrgastro.2017.79
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2017.79
This article is cited by
-
Computational fluid dynamic modeling of the lymphatic system: a review of existing models and future directions
Biomechanics and Modeling in Mechanobiology (2024)
-
Molecular and metabolic orchestration of the lymphatic vasculature in physiology and pathology
Nature Communications (2023)
-
The emerging roles of long noncoding RNAs in lymphatic vascular development and disease
Cellular and Molecular Life Sciences (2023)
-
ADAMTS18+ villus tip telocytes maintain a polarized VEGFA signaling domain and fenestrations in nutrient-absorbing intestinal blood vessels
Nature Communications (2022)
-
Lipid absorption and overall intestinal lymphatic transport are impaired following partial small bowel resection in mice
Scientific Reports (2022)