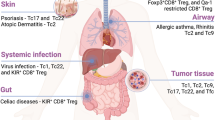
Key Points
-
CD4+ T cells are plastic, being able to both acquire distinct functions to combat specific pathogens and redirect their functions in response to changing circumstances or different pathogens.
-
T cell plasticity is an inherent characteristic of T cell responses that provides advantages to the host for protective immunity and immune control.
-
Extracellular cues from the environment drive T cell reprogramming, but they do so within the framework of cytosolic signalling, metabolic and epigenetic circuitry that is established in the cell to stabilize polarized T cell functions.
-
T cell plasticity is widely observed in immune-based diseases, such as autoimmunity and cancer, and may contribute to disease pathology.
-
There is potential to modulate immunity by targeting key regulatory nodes that programme inflammatory versus regulatory functions in T cells.
Abstract
CD4+ T cells differentiate and acquire distinct functions to combat specific pathogens but can also adapt their functions in response to changing circumstances. Although this phenotypic plasticity can be potentially deleterious, driving immune pathology, it also provides important benefits that have led to its evolutionary preservation. Here, we review CD4+ T cell plasticity by examining the molecular mechanisms that regulate it — from the extracellular cues that initiate and drive cells towards varying phenotypes, to the cytosolic signalling cascades that decipher these cues and transmit them into the cell and to the nucleus, where these signals imprint specific gene expression programmes. By understanding how this functional flexibility is achieved, we may open doors to new therapeutic approaches that harness this property of T cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mosmann, T. R., Cherwinski, H., Bond, M. W., Giedlin, M. A. & Coffman, R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136, 2348–2357 (1986).
Del Prete, G. F. et al. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J. Clin. Invest. 88, 346–350 (1991).
Abbas, A. K., Murphy, K. M. & Sher, A. Functional diversity of helper T lymphocytes. Nature 383, 787–793 (1996).
Burkett, P. R., Meyer zu Horste, G. & Kuchroo, V. K. Pouring fuel on the fire: Th17 cells, the environment, and autoimmunity. J. Clin. Invest. 125, 2211–2219 (2015).
Schmitt, E., Klein, M. & Bopp, T. Th9 cells, new players in adaptive immunity. Trends Immunol. 35, 61–68 (2014).
Crotty, S. T. Follicular helper cell differentiation, function, and roles in disease. Immunity 41, 529–542 (2014).
Wing, J. B. & Sakaguchi, S. Multiple Treg suppressive modules and their adaptability. Front. Immunol. 3, 178 (2012).
Wilson, C. B., Rowell, E. & Sekimata, M. Epigenetic control of T-helper-cell differentiation. Nat. Rev. Immunol. 9, 91–105 (2009).
Zhou, L., Chong, M. M. W. & Littman, D. R. Plasticity of CD4+ T cell lineage differentiation. Immunity 30, 646–655 (2009).
O'Shea, J. J. & Paul, W. E. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 327, 1098–1102 (2010).
Murphy, K. M. & Stockinger, B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat. Immunol. 11, 674–680 (2010).
Bluestone, J. A., Mackay, C. R., O'Shea, J. J. & Stockinger, B. The functional plasticity of T cell subsets. Nat. Rev. Immunol. 9, 811–816 (2009).
Wang, Y. H. et al. A novel subset of CD4+ TH2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J. Exp. Med. 207, 2479–2491 (2010).
Dominguez-Villar, M., Baecher-Allan, C. M. & Hafler, D. A. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat. Med. 17, 673–675 (2011).
Malmhäll, C. et al. Immunophenotyping of circulating T helper cells argues for multiple functions and plasticity of T cells in vivo in humans - possible role in asthma. PLoS ONE 7, e40012 (2012).
Perez, V. L., Lederer, J. A., Lichtman, A. H. & Abbas, A. K. Stability of Th1 and Th2 populations. Int. Immunol. 7, 869–875 (1995).
Koenen, H. J. P. M. et al. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood 112, 2340–2352 (2008).
Yang, X. O. et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity 29, 44–56 (2008).
Lee, Y. K. et al. Late developmental plasticity in the T helper 17 lineage. Immunity 30, 92–107 (2009).
Lohning, M. et al. Long-lived virus-reactive memory T cells generated from purified cytokine-secreting T helper type 1 and type 2 effectors. J. Exp. Med. 205, 53–61 (2008).
Bending, D. et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J. Clin. Invest. 119, 565–572 (2009).
Jager, A., Dardalhon, V., Sobel, R. A., Bettelli, E. & Kuchroo, V. K. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J. Immunol. 183, 7169–7177 (2009).
Lüthje, K. et al. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat. Immunol. 13, 491–498 (2012).
Panzer, M. et al. Rapid in vivo conversion of effector T cells into Th2 Cells during helminth infection. J. Immunol. 188, 615–623 (2012).
Harbour, S. N., Maynard, C. L., Zindl, C. L., Schoeb, T. R. & Weaver, C. T. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc. Natl Acad. Sci. USA 112, 7061–7066 (2015).
Zhou, X. et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 10, 1000–1007 (2009). This study uses fate mapping techniques in mice to show that T Reg cells can lose the expression of FOXP3 and that these 'ex-FOXP3' cells can drive autoimmune diabetes.
Hirota, K. et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol. 12, 255–263 (2011).
Wilhelm, C. et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat. Immunol. 12, 1071–1077 (2011).
Ahlfors, H. et al. IL-22 fate reporter reveals origin and control of IL-22 production in homeostasis and infection. J. Immunol. 193, 4602–4613 (2014).
Becattini, S. et al. T cell immunity. Functional heterogeneity of human memory CD4+ T cell clones primed by pathogens or vaccines. Science 347, 400–406 (2015).
Han, A., Glanville, J., Hansmann, L. & Davis, M. M. Linking T-cell receptor sequence to functional phenotype at the single-cell level. Nat. Biotechnol. 32, 684–692 (2014). References 30 and 31 map the fate of human T cells by combining high-throughput sequencing of TCRs with T cell functional analyses.
Wang, C. et al. CD5L/AIM regulates lipid biosynthesis and restrains Th17 cell pathogenicity. Cell 163, 1413–1427 (2015).
Gaublomme, J. T. et al. Single-Cell genomics unveils critical regulators of Th17 cell pathogenicity. Cell 163, 1400–1412 (2015). References 32 and 33 use single-cell RNA sequencing to show that individual cells within the T H 17 cell subset exhibit heterogeneity in cellular functions from pathogenic to regulatory phenotypes.
O'Shea, J. J. et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu. Rev. Med. 66, 311–328 (2015).
Hegazy, A. N. et al. Interferons direct Th2 cell reprogramming to generate a stable GATA-3+T-bet+ cell subset with combined Th2 and Th1 cell functions. Immunity 32, 116–128 (2010).
Veldhoen, M. et al. Transforming growth factor-β 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 9, 1341–1346 (2008).
Lu, K. T. et al. Functional and epigenetic studies reveal multistep differentiation and plasticity of in vitro-generated and in vivo-derived follicular T helper cells. Immunity 35, 622–632 (2011).
Knosp, C. A. & Johnston, J. A. Regulation of CD4+ T-cell polarization by suppressor of cytokine signalling proteins. Immunology 135, 101–111 (2012).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Zhou, L. et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature 453, 236–240 (2008).
Gagliani, N. et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523, 221–225 (2015). In this study, fate mapping techniques in mice reveal that inflammatory cells can reprogramme into regulatory cells.
Stumhofer, J. S. et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 8, 1363–1371 (2007).
Apetoh, L. et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 11, 854–861 (2010).
Hunter, C. A. & Kastelein, R. Interleukin-27: balancing protective and pathological immunity. Immunity 37, 960–969 (2012).
Laurence, A. et al. STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft-versus-host disease. Immunity 37, 209–222 (2012).
Oldenhove, G. et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity 31, 772–786 (2009).
Koch, M. A. et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 10, 595–602 (2009).
McClymont, S. A. et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J. Immunol. 186, 3918–3926 (2011).
Campbell, D. J. & Koch, M. A. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat. Rev. Immunol. 11, 119–130 (2011).
Feng, T., Cao, A. T., Weaver, C. T., Elson, C. O. & Cong, Y. Interleukin-12 converts Foxp3+ regulatory T cells to interferon-γ-producing Foxp3+ T cells that inhibit colitis. Gastroenterology 140, 2031–2043 (2011).
Fuhrman, C. A. et al. Divergent phenotypes of human regulatory T cells expressing the receptors TIGIT and CD226. J. Immunol. 195, 145–155 (2015).
Tang, Q. et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 28, 687–697 (2008).
Feng, Y. et al. Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell 158, 749–763 (2014).
Klatzmann, D. & Abbas, A. K. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat. Rev. Immunol. 15, 283–294 (2015).
van Panhuys, N., Klauschen, F. & Germain, R. N. T-cell-receptor-dependent signal intensity dominantly controls CD4+ T cell polarization in vivo. Immunity 41, 63–74 (2014).
Yamane, H. & Paul, W. E. Early signaling events that underlie fate decisions of naive CD4+ T cells toward distinct T-helper cell subsets. Immunol. Rev. 252, 12–23 (2013).
Fazilleau, N., McHeyzer-Williams, L. J., Rosen, H. & McHeyzer-Williams, M. G. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat. Immunol. 10, 375–384 (2009).
Tubo, N. J. et al. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell 153, 785–796 (2013).
Sauer, S. et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl Acad. Sci. USA 105, 7797–7802 (2008).
Gottschalk, R. A., Corse, E. & Allison, J. P. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J. Exp. Med. 207, 1701–1711 (2010).
Hsieh, C. S., Lee, H. M. & Lio, C. W. Selection of regulatory T cells in the thymus. Nat. Rev. Immunol. 12, 157–167 (2012).
Ohkura, N. et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity 37, 785–799 (2012). This study identifies key epigenetic features of T Reg cells driven by TCR signals and independent of FOXP3 expression.
Nagaoka, M., Hatta, Y., Kawaoka, Y. & Malherbe, L. P. Antigen signal strength during priming determines effector CD4 T cell function and antigen sensitivity during influenza virus challenge. J. Immunol. 193, 2812–2820 (2014).
Ahmadzadeh, M. & Farber, D. L. Functional plasticity of an antigen-specific memory CD4 T cell population. Proc. Natl Acad. Sci. USA 99, 11802–11807 (2002).
Martinez-Sanchez, M. E., Mendoza, L., Villarreal, C. & Alvarez-Buylla, E. R. A. Minimal regulatory network of extrinsic and intrinsic factors recovers observed patterns of CD4+ T cell differentiation and plasticity. PLoS Comput. Biol. 11, e1004324 (2015).
Moran, A. E. et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 208, 1279–1289 (2011).
Levine, A. G., Arvey, A., Jin, W. & Rudensky, A. Y. Continuous requirement for the TCR in regulatory T cell function. Nat. Immunol. 15, 1070–1078 (2014).
Vahl, J. C. et al. Continuous T cell receptor signals maintain a functional regulatory T cell pool. Immunity 41, 722–736 (2014).
Tang, Q. et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+ CD25+ regulatory T cells. J. Immunol. 171, 3348 (2003).
Zhang, R. et al. An obligate cell-intrinsic function for CD28 in Tregs. J. Clin. Invest. 123, 580–593 (2013).
Bailey-Bucktrout, S. L. et al. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity 39, 949–962 (2013).
Gratz, I. K. et al. Cutting edge: self-antigen controls the balance between effector and regulatory T cells in peripheral tissues. J. Immunol. 192, 1351–1355 (2014).
Laky, K., Evans, S., Perez-Diez, A. & Fowlkes, B. J. Notch signaling regulates antigen sensitivity of naive CD4+ T cells by tuning co-stimulation. Immunity 42, 80–94 (2015).
Bensinger, S. J. et al. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J. Immunol. 172, 5287–5296 (2004).
Buckler, J. L., Liu, X. & Turka, L. A. Regulation of T-cell responses by PTEN. Immunol. Rev. 224, 239–248 (2008).
Crellin, N. K., Garcia, R. V. & Levings, M. K. Altered activation of AKT is required for the suppressive function of human CD4+CD25+ T regulatory cells. Blood 109, 2014–2022 (2007).
Walsh, P. T. et al. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J. Clin. Invest. 116, 2521–2531 (2006).
Patterson, S. J. et al. Cutting edge: PHLPP regulates the development, function, and molecular signaling pathways of regulatory T cells. J. Immunol. 186, 5533–5537 (2011).
Huynh, A. et al. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat. Immunol. 16, 188–196 (2015).
Shrestha, S. et al. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat. Immunol. 16, 178–187 (2015). References 79 and 80 show that uncontrolled AKT activation in T Reg cells drives the instability of FOXP3 expression.
Ouyang, W. et al. Novel Foxo1-dependent transcriptional programs control Treg cell function. Nature 491, 554–559 (2012).
Delgoffe, G. M. et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 501, 252–256 (2013).
Yang, K. et al. T cell exit from quiescence and differentiation into Th2 cells depend on Raptor-mTORC1-mediated metabolic reprogramming. Immunity 39, 1043–1056 (2013).
Delgoffe, G. M. et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 12, 295–303 (2011).
Delgoffe, G. M. et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30, 832–844 (2009).
Lee, K. et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity 32, 743–753 (2010).
Kurebayashi, Y. et al. PI3K-Akt-mTORC1-S6K1/2 axis controls Th17 differentiation by regulating Gfi1 expression and nuclear translocation of RORγ. Cell Rep. 1, 360–373 (2012).
Zeng, H. et al. mTORC1 couples immune signals and metabolic programming to establish Treg-cell function. Nature 499, 485–490 (2013).
Yurchenko, E. et al. Inflammation-driven reprogramming of CD4+Foxp3+ regulatory T cells into pathogenic Th1/Th17 T effectors is abrogated by mTOR inhibition in vivo. PLoS ONE 7, e35572 (2012).
Park, Y. et al. TSC1 regulates the balance between effector and regulatory T cells. J. Clin. Invest. 123, 5165–5178 (2013).
Miskov-Zivanov, N., Turner, M. S., Kane, L. P., Morel, P. A. & Faeder, J. R. The duration of T cell stimulation is a critical determinant of cell fate and plasticity. Sci. Signal. 6, ra97 (2013).
Pearce, E. L., Poffenberger, M. C., Chang, C. H. & Jones, R. G. Fueling immunity: insights into metabolism and lymphocyte function. Science 342, 1242454–1242454 (2013).
Frauwirth, K. A. et al. The CD28 signaling pathway regulates glucose metabolism. Immunity 16, 769–777 (2002).
Macintyre, A. N. et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell. Metab. 20, 61–72 (2014).
Basu, S., Hubbard, B. & Shevach, E. M. Foxp3-mediated inhibition of Akt inhibits Glut1 (glucose transporter 1) expression in human T regulatory cells. J. Leukocyte Biol. 97, 279–283 (2015).
Michalek, R. D. et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186, 3299–3303 (2011).
Gerriets, V. A. et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J. Clin. Invest. 125, 194–207 (2014). References 96 and 97 show that distinct metabolic programmes are required for the functional capacities of T cells and reveal key differences between the metabolic programmes of inflammatory and regulatory T cells.
Furusawa, Y. et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013).
Klysz, D. et al. Glutamine-dependent α-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation. Sci. Signal. 8, ra97 (2015). This study links the disruption in proper control of glycolytic metabolism in T Reg cells to FOXP3 instability.
Nakamura, H. et al. TCR engagement increases hypoxia-inducible factor-1 protein synthesis via rapamycin-sensitive pathway under hypoxic conditions in human peripheral T cells. J. Immunol. 174, 7592–7599 (2005).
Shi, L. Z. et al. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 208, 1367–1376 (2011).
Lee, J. H., Elly, C., Park, Y. & Liu, Y.-C. E3 ubiquitin ligase VHL regulates hypoxia-inducible factor-1α to maintain regulatory T cell stability and suppressive capacity. Immunity 42, 1062–1074 (2015).
Dang, E. V. et al. Control of TH17/Treg balance by hypoxia-inducible factor 1. Cell 146, 772–784 (2011).
Oestreich, K. J. et al. Bcl-6 directly represses the gene program of the glycolysis pathway. Nat. Immunol. 15, 957–964 (2014).
Tamas, P. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J. Exp. Med. 203, 1665–1670 (2006).
MacIver, N. J. et al. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J. Immunol. 187, 4187–4198 (2011).
Blagih, J. et al. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity 42, 41–54 (2015).
Faubert, B. et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell. Metab. 17, 113–124 (2013).
Chang, C.-H. et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 (2013).
Berod, L. et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 20, 1327–1333 (2014).
Williams, C. L. et al. STAT4 and T-bet are required for the plasticity of IFN-γ expression across Th2 ontogeny and influence changes in Ifng promoter DNA methylation. J. Immunol. 191, 678–687 (2013).
Vahedi, G. et al. STATs shape the active enhancer landscape of T cell populations. Cell 151, 981–993 (2012).
Goswami, R. et al. STAT6-dependent regulation of Th9 development. J. Immunol. 188, 968–975 (2012).
Samstein, R. M. et al. Foxp3 exploits a pre-existent enhancer landscape for regulatory T cell lineage specification. Cell 151, 153–166 (2012).
Oestreich, K. J. & Weinmann, A. S. Master regulators or lineage-specifying? Changing views on CD4+ T cell transcription factors. Nat. Rev. Immunol. 12, 799–804 (2012).
Wei, G. et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30, 155–167 (2009).
Shih, H. Y. et al. Transcriptional and epigenetic networks of helper T and innate lymphoid cells. Immunol. Rev. 261, 23–49 (2014).
Fu, W. et al. A multiply redundant genetic switch 'locks in' the transcriptional signature of regulatory T cells. Nat. Immunol. 13, 972–980 (2012).
Lee, T. I. & Young, R. A. Transcriptional regulation and its misregulation in disease. Cell 152, 1237–1251 (2013).
Roychoudhuri, R. et al. BACH2 represses effector programs to stabilize Treg-mediated immune homeostasis. Nature 498, 506–510 (2013).
Wang, Y. et al. The transcription factors T-bet and Runx are required for the ontogeny of pathogenic interferon-γ-producing T helper 17 cells. Immunity 40, 355–366 (2014).
Sekiya, T. et al. Suppression of Th2 and Tfh immune reactions by Nr4a receptors in mature T reg cells. J. Exp. Med. 212, 1623–1640 (2015). This paper reveals the essential role of additional transcription factors that collaborate with FOXP3 to maintain the T Reg cell transcriptional programme.
Brown, C. C. et al. Retinoic acid is essential for Th1 cell lineage stability and prevents transition to a Th17 cell program. Immunity 42, 499–511 (2015).
Park, B. V. & Pan, F. The role of nuclear receptors in regulation of Th17/Treg biology and its implications for diseases. Cell. Mol. Immunol. 12, 533–542 (2015).
Bird, J. et al. Helper T cell differentiation is controlled by the cell cycle. Immunity 9, 229–237 (1998).
Agarwal, S. & Rao, A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity 9, 765–775 (1998).
Grogan, J. L. et al. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity 14, 205–215 (2001).
Avni, O. et al. TH cell differentiation is accompanied by dynamic changes in histone acetylation of cytokine genes. Nat. Immunol. 3, 643–651 (2002).
Hojfeldt, J. W., Agger, K. & Helin, K. Histone lysine demethylases as targets for anticancer therapy. Nat. Rev. Drug Discov. 12, 917–930 (2013).
Pastor, W. A., Aravind, L. & Rao, A. TETonic shift: biological roles of TET proteins in DNA demethylation and transcription. Nat. Rev. Mol. Cell Biol. 14, 341–356 (2013).
Zhang, F. T helper type 1-specific Brg1 recruitment and remodeling of nucleosomes positioned at the IFN-γ promoter are Stat4 dependent. J. Exp. Med. 203, 1493–1505 (2006).
Schones, D. E. et al. Dynamic regulation of nucleosome positioning in the human genome. Cell 132, 887–898 (2008).
Wang, Z. et al. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell 138, 1019–1031 (2009).
Ichiyama, K. et al. The methylcytosine dioxygenase Tet2 promotes DNA demethylation and activation of cytokine gene expression in T Cells. Immunity 42, 613–626 (2015).
Onodera, A. & Nakayama, T. Epigenetics of T cells regulated by Polycomb/Trithorax molecules. Trends Mol. Med. 21, 330–340 (2015).
Miller, S. A., Huang, A. C., Miazgowicz, M. M., Brassil, M. M. & Weinmann, A. S. Coordinated but physically separable interaction with H3K27- demethylase and H3K4-methyltransferase activities are required for T-box protein-mediated activation of developmental gene expression. Genes Dev. 22, 2980–2993 (2008).
Bettini, M. L. et al. Loss of epigenetic modification driven by the Foxp3 transcription factor leads to regulatory T cell insufficiency. Immunity 36, 717–730 (2012).
Wang, L. et al. Mbd2 promotes Foxp3 demethylation and T-regulatory-cell function. Mol. Cell. Biol. 33, 4106–4115 (2013).
Yang, R. et al. Hydrogen sulfide promotes Tet1- and Tet2-mediated Foxp3 demethylation to drive regulatory T cell differentiation and maintain immune homeostasis. Immunity 43, 251–263 (2015). References 139 and 140 reveal that DNA demethylation by TET proteins is crucial for the maintenance of FOXP3 expression and T Reg cell stability.
Liu, Y. et al. Two histone/protein acetyltransferases, CBP and p300, are indispensable for Foxp3+ T-regulatory cell development and function. Mol. Cell. Biol. 34, 3993–4007 (2014).
Yamashita, M. et al. Crucial role of MLL for the maintenance of memory T helper type 2 cell responses. Immunity 24, 611–622 (2006).
Collier, S. P., Collins, P. L., Williams, C. L., Boothby, M. R. & Aune, T. M. Cutting edge: influence of Tmevpg1, a long intergenic noncoding RNA, on the expression of Ifng by Th1 cells. J. Immunol. 189, 2084–2088 (2012).
Gomez, J. A. et al. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-γ locus. Cell 152, 743–754 (2013).
Satpathy, A. T. & Chang, H. Y. Long noncoding RNA in hematopoiesis and immunity. Immunity 42, 792–804 (2015).
van den Ham, H. J. & de Boer, R. J. Cell division curtails helper phenotype plasticity and expedites helper T-cell differentiation. Immunol. Cell Biol. 90, 860–868 (2012).
Thomas, R. M., Gamper, C. J., Ladle, B. H., Powell, J. D. & Wells, A. D. De novo DNA methylation is required to restrict T helper lineage plasticity. J. Biol. Chem. 287, 22900–22909 (2012).
Liu, B., Tahk, S., Yee, K. M., Fan, G. & Shuai, K. The ligase PIAS1 restricts natural regulatory T cell differentiation by epigenetic repression. Science 330, 521–525 (2010).
Busslinger, M. & Tarakhovsky, A. Epigenetic control of immunity. Cold Spring Harb. Perspect. Biol. 6, a019307 (2014).
Wang, L. et al. FOXP3+ regulatory T cell development and function require histone/protein deacetylase 3. J. Clin. Invest. 125, 1111–1123 (2015).
Arvey, A. et al. Inflammation-induced repression of chromatin bound by the transcription factor Foxp3 in regulatory T cells. Nat. Immunol. 15, 580–587 (2014).
DuPage, M. et al. The chromatin-modifying enzyme Ezh2 is critical for the maintenance of regulatory T cell identity after activation. Immunity 42, 227–238 (2015). References 151 and 152 together show that EZH2 activity in activated T Reg cells is required for T Reg cell function and stability by maintaining the FOXP3-driven transcriptional programme.
Tumes, D. J. et al. The polycomb protein Ezh2 regulates differentiation and plasticity of CD4+ T helper type 1 and type 2 cells. Immunity 39, 819–832 (2013).
Zhang, Y. et al. The polycomb repressive complex 2 governs life and death of peripheral T cells. Blood 124, 737–749 (2014).
Escobar, T. M. et al. miR-155 activates cytokine gene expression in Th17 cells by regulating the DNA-binding protein Jarid2 to relieve polycomb-mediated repression. Immunity 40, 865–879 (2014).
Li, Q. et al. Critical role of histone demethylase Jmjd3 in the regulation of CD4+ T-cell differentiation. Nat. Commun. 5, 5780 (2014).
Allan, R. S. et al. An epigenetic silencing pathway controlling T helper 2 cell lineage commitment. Nature 487, 249–253 (2012).
Antignano, F. et al. Methyltransferase G9A regulates T cell differentiation during murine intestinal inflammation. J. Clin. Invest. 124, 1945–1955 (2014).
Huang, C. et al. Cutting edge: a novel, human-specific interacting protein couples FOXP3 to a chromatin-remodeling complex that contains KAP1/TRIM28. J. Immunol. 190, 4470–4473 (2013).
Xu, K. et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science 338, 1465–1469 (2012).
Kim, E. et al. Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells. Cancer Cell 23, 839–852 (2013).
Badeaux, A. I. & Shi, Y. Emerging roles for chromatin as a signal integration and storage platform. Nat. Rev. Mol. Cell Biol. 14, 211–224 (2013).
Baumjohann, D. & Ansel, K. M. MicroRNA-mediated regulation of T helper cell differentiation and plasticity. Nat. Rev. Immunol. 13, 666–678 (2013).
Long, S. A. et al. Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4+CD25+ regulatory T-cells of type 1 diabetic subjects. Diabetes 59, 407–415 (2010).
Carbone, F. et al. Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat. Med. 20, 69–74 (2013).
Bending, D. et al. Hypomethylation at the regulatory T cell-specific demethylated region in CD25hi T cells is decoupled from FOXP3 expression at the inflamed site in childhood arthritis. J. Immunol. 193, 2699–2708 (2014).
Rivas, M. N. et al. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity 42, 512–523 (2015).
Komatsu, N. et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 20, 62–68 (2014).
Blatner, N. R. et al. Expression of RORγt marks a pathogenic regulatory T cell subset in human colon cancer. Sci. Transl Med. 4, 164ra159 (2012). This paper shows that selectively targeting the RORγt+ T Reg cell subset that promoted cancer was a successful strategy to treat the disease.
McGeachy, M. J. et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 10, 314–324 (2009).
Hirota, K. et al. Plasticity of TH17 cells in Peyer's patches is responsible for the induction of T cell–dependent IgA responses. Nat. Immunol. 14, 372–379 (2013).
Araya, N. et al. HTLV-1 induces a Th1-like state in CD4+CCR4+ T cells. J. Clin. Invest. 124, 3431–3442 (2014).
Bluestone, J. A., Crellin, N. & Trotta, E. IL-2: change structure... change function. Immunity 42, 779–781 (2015).
Herold, K. C. et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N. Engl. J. Med. 346, 1692–1698 (2002).
Penaranda, C., Tang, Q. & Bluestone, J. A. Anti-CD3 therapy promotes tolerance by selectively depleting pathogenic cells while preserving regulatory T cells. J. Immunol. 187, 2015–2022 (2011).
Littman, D. R. Releasing the brakes on cancer immunotherapy. Cell 162, 1186–1190 (2015).
Long, S. A. et al. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs β-cell function. Diabetes 61, 2340–2348 (2012).
Ali, K. et al. Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer. Nature 510, 407–411 (2014). This study shows that targeting a specific PI3K isoform used in T Reg cells selectively destabilizes T Reg cells and promotes antitumour T cell responses.
Putnam, A. L. et al. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes 58, 652–662 (2009).
Fischbach, M. A., Bluestone, J. A. & Lim, W. A. Cell-based therapeutics: the next pillar of medicine. Sci. Transl Med. 5, 179ps177 (2013).
Guedan, S. et al. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood 124, 1070–1080 (2014).
Yin, Y. et al. Normalization of CD4+ T cell metabolism reverses lupus. Sci. Transl Med. 7, 274ra218 (2015). In this paper, broadly inhibiting glycolytic metabolism is shown to reverse autoimmune lupus.
Chen, Z. et al. The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity 39, 272–285 (2013).
Yang, J. et al. Cutting edge: ubiquitin-specific protease 4 promotes Th17 cell function under inflammation by deubiquitinating and stabilizing RORγt. J. Immunol. 194, 4094–4097 (2015).
Jang, E. J., Park, H. R., Hong, J. H. & Hwang, E. S. Lysine 313 of T-box is crucial for modulation of protein stability, DNA binding, and threonine phosphorylation of T-bet. J. Immunol. 190, 5764–5770 (2013).
Beier, U. H. et al. Sirtuin-1 targeting promotes Foxp3+ T-regulatory cell function and prolongs allograft survival. Mol. Cell. Biol. 31, 1022–1029 (2011).
Kwon, H. S. et al. Three novel acetylation sites in the Foxp3 transcription factor regulate the suppressive activity of regulatory T cells. J. Immunol. 188, 2712–2721 (2012).
Lim, H. W. et al. SIRT1 deacetylates RORγt and enhances Th17 cell generation. J. Exp. Med. 212, 607–617 (2015).
Peng, D. et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 527, 249–253 (2015). This paper reveals that EZH2 activity in tumour cells can shape the immune microenvironment of tumours by controlling the expression of chemokines.
Liu, Y. et al. Inhibition of p300 impairs Foxp3+ T regulatory cell function and promotes antitumor immunity. Nat. Med. 19, 1173–1177 (2013).
Bandukwala, H. S. et al. Selective inhibition of CD4+ T-cell cytokine production and autoimmunity by BET protein and c-Myc inhibitors. Proc. Natl Acad. Sci. USA 109, 14532–14537 (2012).
Fu, W. et al. Epigenetic modulation of type-1 diabetes via a dual effect on pancreatic macrophages and β cells. eLife 3, e04631 (2014). References 190–192 collectively show that targeting epigenetic enzymes with systemic drugs can have selective immunomodulatory activities to ameliorate cancer or autoimmune disease.
Tsuji, M. et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science 323, 1488–1492 (2009).
Sharma, M. D. et al. An inherently bifunctional subset of Foxp3+ T helper cells is controlled by the transcription factor Eos. Immunity 38, 998–1012 (2013).
Neumann, C. et al. Role of Blimp-1 in programing Th effector cells into IL-10 producers. J. Exp. Med. 211, 1807–1819 (2014).
Esplugues, E. et al. Control of TH17 cells occurs in the small intestine. Nature 475, 514–518 (2011).
Califano, D. et al. Diverting T helper cell trafficking through increased plasticity attenuates autoimmune encephalomyelitis. J. Clin. Invest. 124, 174–187 (2013).
Heinemann, C. et al. IL-27 and IL-12 oppose pro-inflammatory IL-23 in CD4+ T cells by inducing Blimp1. Nat. Commun. 5, 1–13 (2014).
Umetsu, D. T. et al. Functional heterogeneity among human inducer T cell clones. J. Immunol. 140, 4211–4216 (1988).
Maggi, E. et al. Profiles of lymphokine activities and helper function for IgE in human T cell clones. Eur. J. Immunol. 18, 1045–1050 (1988).
Murphy, E. et al. Reversibility of T helper 1 and 2 populations is lost after long-term stimulation. J. Exp. Med. 183, 901–913 (1996).
Komatsu, N. et al. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc. Natl Acad. Sci. USA 106, 1903–1908 (2009).
Duarte, J. H., Zelenay, S., Bergman, M.-L., Martins, A. C. & Demengeot, J. Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur. J. Immunol. 39, 948–955 (2009).
Acknowledgements
The authors thank A. Abbas, K. M. Ansel, R. Locksley, L. Turka and members of the laboratory for their helpful comments and feedback during the preparation of this Review. This work was supported by the Sean N. Parker Autoimmune Research Laboratory, the Juvenile Diabetes Research Foundation (JDRF; 2-SRA-2014-150), the US National Institutes of Health (NIH; R01 AI046643, U01 AI102011) and the Helen Hay Whitney Foundation (to M.D.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- mTOR complex 1
-
(mTORC1). A complex consisting of: mammalian target of rapamycin (mTOR), which is a serine/threonine kinase; regulatory-associated protein of mTOR (RAPTOR); proline-rich AKT1 substrate of 40 kDa (PRAS40), which is an mTORC1 inhibitor; mLST8, which is of unknown function; and DEP domain-containing mTOR-interacting protein (DEPTOR), which is an mTOR inhibitor.
- mTORC2
-
A complex composed of mammalian target of rapamycin (mTOR), mLST8 and the adaptor proteins rapamycin-insensitive companion of mTOR (RICTOR) and stress-activated MAP kinase-interacting protein 1 (SIN1).
- Tricarboxylic acid cycle
-
(TCA cycle). This pathway (also known as the Krebs cycle or citric acid cycle) catalyses the oxidation of acetyl-CoA (from glucose or fatty acids or indirectly from amino acids) to generate NADH and FADH, which fuel the electron transport chain and thereby oxidative phosphorylation and ATP production. It also serves as a source of precursors for amino acid and lipid synthesis.
- Innate lymphoid cells
-
Lymphoid cells that do not express unique antigen receptors derived from gene rearrangement or cell-surface markers that are characteristic of other immune cell lineages. However, in response to innate tissue-derived signals, they secrete cytokines that are associated with T helper cell subsets. They have important roles in innate immune responses to infectious microorganisms and in lymphoid tissue formation.
- Long non-coding RNAs
-
(lncRNAs). A subset of non-coding RNAs that are greater than 200 nucleotides in length and distinct from other non-coding RNAs, such as tRNAs, rRNAs, snRNAs, small nucleolar RNAs (snoRNAs), and microRNAs. Although they are known to regulate gene expression, the mechanisms by which this is achieved is an active area of investigation.
- Chimeric antigen receptor
-
(CAR). An artificial T cell receptor construct that consists of an extracellular single-chain antibody fragment that functions as the antigen-binding domain, together with transmembrane and intracellular signalling domains from the T cell receptor CD3 ζ-chain and/or from a co-stimulatory molecule such as CD28.
- BET inhibitors
-
Inhibitors that bind the bromodomain and extraterminal (BET) motif of several bromodomain-containing proteins (BRDs), blocking their interaction with acetylated lysines on histones and preventing their promotion of transcription.
Rights and permissions
About this article
Cite this article
DuPage, M., Bluestone, J. Harnessing the plasticity of CD4+ T cells to treat immune-mediated disease. Nat Rev Immunol 16, 149–163 (2016). https://doi.org/10.1038/nri.2015.18
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri.2015.18
This article is cited by
-
TIGIT reverses IFN-α-promoted Th1-like Tregs via in-sequence effects dependent on STAT4
Arthritis Research & Therapy (2023)
-
Innate lymphoid cells and innate-like T cells in cancer — at the crossroads of innate and adaptive immunity
Nature Reviews Cancer (2023)
-
The broad spectrum of pathogenic autoreactivity
Nature Reviews Immunology (2023)
-
Brain regulatory T cells
Nature Reviews Immunology (2023)
-
Neuroinflammation and Immune Dysfunction in the Mechanisms of Development of Parkinson’s Disease
Neuroscience and Behavioral Physiology (2023)