Key Points
-
Dendritic cells (DCs) express Toll-like receptors (TLRs) and C-type lectins that interact with pathogens.
-
C-type lectins interact with specific carbohydrate structures on pathogens to internalize pathogens for degradation in lysosomal compartments to enhance antigen processing and presentation.
-
TLRs recognize molecular patterns on pathogens that leads to DC activation, expression of co-stimulatory molecules and the production of inflammatory cytokines.
-
Different DC-subsets express different sets of TLRs and C-type lectins to orchestrate specific immune responses.
-
C-type lectins recognize self and non-self antigens, and can function as antigen receptors, adhesion receptors and signalling receptors. So far, only little is known about the antigen specificity of C-type lectins with the exception of the DC-specific C-type lectin DC-SIGN.
-
Cell- and tissue-specific glycosylation of a glycoprotein regulates the expression of carbohydrate residues and recognition by specific C-type lectins.
-
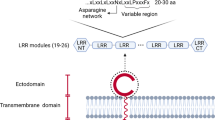
The C-type lectin DC-SIGN recognizes a wide variety of pathogens such as viruses, bacteria, yeast and parasites through the binding of mannose or Lewis-x carbohydrate structures.
-
DC-SIGN also functions as an adhesion receptor through binding of intercellular adhesion molecule 2 (ICAM2) and ICAM3.
-
Pathogens such as HIV-1 and Mycobacteria tuberculosis target DC-SIGN to escape immune surveillance.
-
HIV-1 targets DC-SIGN to hide in DCs and for efficient transmission to T cells, as DC-SIGN functions as a trans-receptor that efficiently presents infectious virus to target T cells.
-
Mycobacteria tuberculosis subverts DCs by targeting DC-SIGN, leading to altered signalling events that inhibit TLR signalling and suppress DC maturation.
-
The balance between TLRs and C-type lectins triggering by pathogens might be instrumental in the final immune response — immune activation or immune suppression.
Abstract
Dendritic cells (DCs) are crucial in the defence against pathogens. Invading pathogens are recognized by Toll-like receptors (TLRs) and receptors such as C-type lectins expressed on the surface of DCs. However, it is becoming evident that some pathogens, including viruses, such as HIV-1, and non-viral pathogens, such as Mycobacterium tuberculosis, subvert DC functions to escape immune surveillance by targeting the C-type lectin DC-SIGN (DC-specific intercellular adhesion molecule-grabbing nonintegrin). Notably, these pathogens misuse DC-SIGN by distinct mechanisms that either circumvent antigen processing or alter TLR-mediated signalling, skewing T-cell responses. This implies that adaptation of pathogens to target DC-SIGN might support pathogen survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Banchereau, J. & Steinman, R. M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Mellman, I., Turley, S. J. & Steinman, R. M. Antigen processing for amateurs and professionals. Trends Cell. Biol. 8, 231–237 (1998).
de Jong, E. C. et al. Microbial compounds selectively induce TH1 cell-promoting or TH2 cell-promoting dendritic cells in vitro with diverse TH cell-polarizing signals. J. Immunol. 168, 1704–1709 (2002).
d'Ostiani, C. F. et al. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 191, 1661–1674 (2000).
Janeway, C. A. Jr & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002).
Underhill, D. M. & Ozinsky, A. Toll-like receptors: key mediators of microbe detection. Curr. Opin. Immunol. 14, 103–110 (2002).
Thoma-Uszynski, S. et al. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science 291, 1544–1547 (2001).
Weis, W. I., Taylor, M. E. & Drickamer, K. The C-type lectin superfamily in the immune system. Immunol. Rev. 163, 19–34 (1998).
Akira, S. & Hemmi, H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol. Lett. 85, 85–95 (2003).
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000).
Figdor, C. G., van Kooyk, Y. & Adema, G. J. C-type lectin receptors on dendritic cells and Langerhans cells. Nature Rev. Immunol. 2, 77–84 (2002).
Engering, A. et al. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J. Immunol. 168, 2118–2126 (2002).
Sallusto, F., Cella, M., Danieli, C. & Lanzavecchia, A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182, 389–400 (1995).
Mahnke, K. et al. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J. Cell. Biol. 151, 673–684 (2000).
Geijtenbeek, T. B. H. et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100, 575–585 (2000). The first paper that identified DC-SIGN (dendritic cell (DC)-specific intercellular adhesion molecule-grabbing nonintegrin) as a DC specific C-type lectin that regulates DC-induced T-cell proliferation.
Dzionek, A. et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon α/β induction. J. Exp. Med. 194, 1823–1834 (2001).
Willment, J. A., Gordon, S. & Brown, G. D. Characterization of the human β-glucan receptor and its alternatively spliced isoforms. J. Biol. Chem. 276, 43818–43823 (2001).
Bates, E. E. et al. APCs express DCIR, a novel C-type lectin surface receptor containing an immunoreceptor tyrosine-based inhibitory motif. J. Immunol. 163, 1973–1983 (1999).
Ryan, E. J. et al. Dendritic cell-associated lectin-1: a novel dendritic cell-associated, C-type lectin-like molecule enhances T cell secretion of IL-4. J. Immunol. 169, 5638–5648 (2002).
Colonna, M., Samaridis, J. & Angman, L. Molecular characterization of two novel C-type lectin-like receptors, one of which is selectively expressed in human dendritic cells. Eur. J. Immunol. 30, 697–704 (2000).
Valladeau, J. et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity 12, 71–81 (2000).
Valladeau, J. et al. Immature human dendritic cells express asialoglycoprotein receptor isoforms for efficient receptor-mediated endocytosis. J. Immunol. 167, 5767–5774 (2001).
Suzuki, N., Yamamoto, K., Toyoshima, S., Osawa, T. & Irimura, T. Molecular cloning and expression of cDNA encoding human macrophage C-type lectin. Its unique carbohydrate binding specificity for Tn antigen. J. Immunol. 156, 128–135 (1996).
Kammerer, U. et al. Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy. Am. J. Pathol. 162, 887–896 (2003).
Relloso, M. et al. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-β, and anti-inflammatory agents. J. Immunol. 168, 2634–2643 (2002).
Jarrossay, D., Napolitani, G., Colonna, M., Sallusto, F. & Lanzavecchia, A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 31, 3388–3393 (2001).
Kadowaki, N. et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194, 863–869 (2001). This paper highlights DC-subset-specific expression of Toll-like receptors (TLRs) and tailored immune responses.
Liu, Y. J. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell 106, 259–262 (2001).
Geijtenbeek, T. B. et al. Identification of different binding sites in the dendritic cell-specific receptor DC-SIGN for intercellular adhesion molecule 3 and HIV-1. J. Biol. Chem. 277, 11314–11320 (2002).
Iobst, S. T., Wormald, M. R., Weis, W. I., Dwek, R. A. & Drickamer, K. Binding of sugar ligands to Ca2+-dependent animal lectins. I. Analysis of mannose binding by site-directed mutagenesis and NMR. J. Biol. Chem. 269, 15505–15511 (1994).
Higashi, N. et al. Human macrophage lectin specific for galactose/N-acetylgalactosamine is a marker for cells at an intermediate stage in their differentiation from monocytes into macrophages. Int. Immunol. 14, 545–554 (2002).
Higashi, N. et al. The macrophage C-type lectin specific for galactose/N-acetylgalactosamine is an endocytic receptor expressed on monocyte-derived immature dendritic cells. J. Biol. Chem. 277, 20686–20693 (2002).
Stambach, N. S. & Taylor, M. E. Characterization of carbohydrate recognition by langerin, a C-type lectin of Langerhans cells. Glycobiology 13, 401–410 (2003).
Frison, N. et al. Oligolysine-based oligosaccharide clusters: selective recognition and endocytosis by the mannose receptor and dendritic cell-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin. J. Biol. Chem. 278, 23922–23929 (2003).
Mitchell, D. A., Fadden, A. J. & Drickamer, K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J. Biol. Chem. 276, 28939–28945 (2001).
Martinez-Pomares, L., Linehan, S. A., Taylor, P. R. & Gordon, S. Binding properties of the mannose receptor. Immunobiol. 204, 527–535 (2001).
Avrameas, A. et al. Expression of a mannose/fucose membrane lectin on human dendritic cells. Eur. J. Immunol. 26, 394–400 (1996).
Weis, W. I. & Drickamer, K. Trimeric structure of a C-type mannose-binding protein. Structure 2, 1227–1240 (1994).
Feinberg, H., Mitchell, D. A., Drickamer, K. & Weis, W. I. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294, 2163–2166 (2001). This paper describes the mechanism of carbohydrate recognition by DC-SIGN.
Drickamer, K. Increasing diversity of animal lectin structures. Curr. Opin. Struct. Biol. 5, 612–616 (1995).
Kato, M. et al. Expression of multilectin receptors and comparative FITC-dextran uptake by human dendritic cells. Int. Immunol. 12, 1511–1519 (2000).
Engering, A., Geijtenbeek, T. B. & van Kooyk, Y. Immune escape through C-type lectins on dendritic cells. Trends Immunol. 23, 480–485 (2002).
Hawiger, D. et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194, 769–779 (2001).
Bonifaz, L. et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 196, 1627–1638 (2002). This paper shows that in vivo targeting of C-type lectin receptors might lead to tolerance induction.
Prigozy, T. I. et al. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity 6, 187–197 (1997).
Steinman, R. M. DC-SIGN: a guide to some mysteries of dendritic cells. Cell 100, 491–494 (2000).
Apostolopoulos, V., Pietersz, G. A., Gordon, S., Martinez-Pomares, L. & McKenzie, I. F. Aldehyde-mannan antigen complexes target the MHC class I antigen-presentation pathway. Eur. J. Immunol. 30, 1714–1723 (2000).
Apostolopoulos, V., Barnes, N., Pietersz, G. A. & McKenzie, I. F. Ex vivo targeting of the macrophage mannose receptor generates anti-tumor CTL responses. Vaccine 18, 3174–3184 (2000).
Lee, B. et al. Cis expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J. Virol. 75, 12028–12038 (2001).
Gordon, S. Pattern recognition receptors: doubling up for the innate immune response. Cell 111, 927–930 (2002). A hypothesis of the innate immune function of pattern recognition receptors expressed by DCs and macrophages is presented in this paper.
van Kooyk, Y. & Geijtenbeek, T. B. A novel adhesion pathway that regulates dendritic cell trafficking and T cell interactions. Immunol. Rev. 186, 47–56 (2002).
Monks, C. R., Freiberg, B. A., Kupfer, H., Sciaky, N. & Kupfer, A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395, 82–86 (1998).
Grakoui, A. et al. The immunological synapse: a molecular machine controlling T cell activation. Science 285, 221–227 (1999).
Dustin, M. L. and Chan, A. C. Signaling takes shape in the immune system. Cell 103, 283–294 (2000).
Lowe, J. B. Glycosylation in the control of selectin counter-receptor structure and function. Immunol. Rev. 186, 19–36 (2002).
Vestweber, D. Ligand-specificity of the selectins. J. Cell. Biochem. 61, 585–591 (1996).
de Fougerolles, A. R., Diamond, M. S. & Springer, T. A. Heterogenous glycosylation of ICAM-3 and lack of interaction with Mac-1 and p150,95. Eur. J. Immunol. 25, 1008–1012 (1995).
Funatsu, O. et al. Structural study of N-linked oligosaccharides of human intercellular adhesion molecule-3 (CD50). Eur. J. Biochem. 268, 1020–1029 (2001).
Daniels, M. A., Hogquist, K. A. & Jameson, S. C. Sweet 'n' sour: the impact of differential glycosylation on T cell responses. Nature Immunol. 3, 903–910 (2002).
Appelmelk, B. J. et al. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J. Immunol. 170, 1635–1639 (2003). This paper identifies the carbohydrate specificity of DC-SIGN and the identification of large variety of pathogens that interact with DC-SIGN.
Kogelberg, H., Montero, E., Bay, S., Lawson, A. M. & Feizi, T. Re-evaluation of monosaccharide binding property of recombinant soluble carbohydrate recognition domain of the natural killer cell receptor NKR-P1A. J. Biol. Chem. 274, 30335–30336 (1999).
Fukui, S., Feizi, T., Galustian, C., Lawson, A. M. & Chai, W. Oligosaccharide microarrays for high-throughput detection and specificity assignments of carbohydrate–protein interactions. Nature Biotechnol. 20, 1011–1017 (2002).
Van Die, I. et al. The dendritic cell specific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis-x. Glycobiology 13, 471–478 (2003).
Curtis, B. M., Scharnowske, S. & Watson, A. J. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc. Natl Acad. Sci. USA 89, 8356–8360 (1992).
Geijtenbeek, T. B. H. et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100, 587–597 (2000). The first paper that described the molecular mechanism by which DCs transmit HIV-1.
Pohlmann, S., Baribaud, F. & Doms, R. W. DC-SIGN and DC-SIGNR: helping hands for HIV. Trends Immunol. 22, 643–646 (2001).
Sol-Foulon, N. et al. HIV-1 Nef-induced upregulation of DC-SIGN in dendritic cells promotes lymphocyte clustering and viral spread. Immunity 16, 145–155 (2002).
Geijtenbeek, T. B. et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197, 7–17 (2003). This paper shows the mechanism by which mycobacteria escape immune surveillance by modulating TLR signalling through targeting DC-SIGN.
Colmenares, M., Puig-Kroger, A., Pello, O. M., Corbi, A. L. & Rivas, L. Dendritic cell (DC)-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN, CD209), a C-type surface lectin in human DCs, is a receptor for Leishmania amastigotes. J. Biol. Chem. 277, 36766–36769 (2002).
Alvarez, C. P. et al. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 76, 6841–6844 (2002).
Tailleux, L. et al. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 197, 121–127 (2003). This paper identifies DC-SIGN as a target for Mycobacterium tuberculosis.
Halary, F. et al. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity 17, 653–664 (2002).
Navarro-Sanchez, E. et al. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO rep. 4, 1–6 (2003).
Tassaneetrithep, B. et al. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 197, 823–829 (2003).
Gardner, J. P. et al. L-SIGN (CD 209L) is a liver-specific capture receptor for hepatitis C virus. Proc. Natl Acad. Sci. USA 100, 4498–4503 (2003).
Pohlmann, S. et al. Hepatitis C virus glycoproteins interact with DC-SIGN and DC-SIGNR. J. Virol. 77, 4070–4080 (2003).
Lozach, P. Y. et al. DC-SIGN and L-SIGN are high–affinity binding receptors for hepatitis C virus glycoprotein E2. J. Biol. Chem. 278, 20358–20366 (2003).
Simmons, G. et al. DC-SIGN and DC-SIGNR bind ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology 305, 115–123 (2003).
Lue, J. et al. Addition of a single gp120 glycan confers increased binding to dendritic cell-specific ICAM-3-grabbing nonintegrin and neutralization escape to human immunodeficiency virus type 1. J. Virol. 76, 10299–10306 (2002).
Lin, C. L. et al. Macrophage-tropic HIV induces and exploits dendritic cell chemotaxis. J. Exp. Med. 192, 587–594 (2000).
Kwon, D. S., Gregorio, G., Bitton, N., Hendrickson, W. A. & Littman, D. R. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16, 135–144 (2002). The first paper to identify that internalization of HIV-1 is required for the infection of T cells by DCs.
Hong, P. W. et al. Human immunodeficiency virus envelope (gp120) binding to DC-SIGN and primary dendritic cells is carbohydrate dependent but does not involve 2G12 or cyanovirin binding sites: implications for structural analyses of gp120–DC-SIGN binding. J. Virol. 76, 12855–12865 (2002).
Maeda, N. et al. The cell surface receptor DC-SIGN discriminates between Mycobacterium species through selective recognition of the mannose caps on lipoarabinomannan. J. Biol. Chem. 278, 5513–5516 (2003).
Cambi, A. et al. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur. J. Immunol. 33, 532–538 (2003).
Okano, M., Satoskar, A. R., Nishizaki, K., Abe, M. & Harn, D. A. Jr. Induction of TH2 responses and IgE is largely due to carbohydrates functioning as adjuvants on Schistosoma mansoni egg antigens. J. Immunol. 163, 6712–6717 (1999).
Cameron, P. U. et al. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257, 383–387 (1992).
Pope, M. et al. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell 78, 389–398 (1994).
Jameson, B. et al. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J. Virol. 76, 1866–1875 (2002).
Kammerer, U. et al. Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy. Am. J. Pathol. 162, 887–896 (2003).
te Velde, A. A. et al. Increased expression of DC-SIGN+ IL-12+IL-18+ and CD83+IL-12−IL-18− dendritic cell populations in the colonic mucosa of patients with Crohn's disease. Eur. J. Immunol. 33, 143–151 (2003).
Engering, A., Van Vliet, S. J., Geijtenbeek, T. B. & van Kooyk, Y. Subset of DC-SIGN+ dendritic cells in human blood transmits HIV-1 to T lymphocytes. Blood 100, 1780–1786 (2002).
Geijtenbeek, T. B., Engering, A. & van Kooyk, Y. DC-SIGN, a C-type lectin on dendritic cells that unveils many aspects of dendritic cell biology. J. Leukoc. Biol. 71, 921–931 (2002).
Baribaud, F., Doms, R. W. & Pohlmann, S. The role of DC-SIGN and DC-SIGNR in HIV and Ebola virus infection: can potential therapeutics block virus transmission and dissemination? Expert Opin. Ther. Targets 6, 423–431 (2002).
Piguet, V. et al. Nef-induced CD4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of β-COP in endosomes. Cell 97, 63–73 (1999).
Granelli-Piperno, A., Delgado, E., Finkel, V., Paxton, W. & Steinman, R. M. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J. Virol. 72, 2733–2737 (1998).
Granelli-Piperno, A., Finkel, V., Delgado, E. & Steinman, R. M. Virus replication begins in dendritic cells during the transmission of HIV-1 from mature dendritic cells to T cells. Curr. Biol. 9, 21–29 (1999).
Nobile, C., Moris, A., Porrot, F., Sol-Foulon, N. & Schwartz, O. Inhibition of human immunodeficiency virus type 1 Env-mediated fusion by DC-SIGN. J. Virol. 77, 5313–5323 (2003).
Baribaud, F. et al. Functional and antigenic characterization of human, rhesus macaque, pigtailed macaque, and murine dc-sign. J. Virol. 75, 10281–10289 (2001).
Geijtenbeek, T. B. et al. Rhesus macaque and chimpanzee DC-SIGN act as HIV/SIV gp120 trans-receptors, similar to human DC-SIGN. Immunol. Lett. 79, 101–107 (2001).
Yu Kimata, M. T. et al. Capture and transfer of simian immunodeficiency virus by macaque dendritic cells is enhanced by DC-SIGN. J. Virol. 76, 11827–11836 (2002).
Schwartz, A. J., Alvarez, X. & Lackner, A. A. Distribution and immunophenotype of DC-SIGN-expressing cells in SIV-infected and uninfected macaques. AIDS Res. Hum. Retroviruses 18, 1021–1029 (2002).
Bobardt, M. D. et al. Syndecan captures, protects, and transmits HIV to T lymphocytes. Immunity 18, 27–39 (2003). This report shows that other non-C-type lectins can function similarly to DC-SIGN as a trans -receptor for HIV-1.
Pohlmann, S. et al. DC-SIGN interactions with human immunodeficiency virus: virus binding and transfer are dissociable functions. J. Virol. 75, 10523–10526 (2001).
Baribaud, F., Pohlmann, S. & Doms, R. W. The role of DC-SIGN and DC-SIGNR in HIV and SIV attachment, infection, and transmission. Virology 286, 1–6 (2001).
McDonald, D. et al. Recruitment of HIV and its receptors to dendritic cell–T cell junctions. Science 300, 1295–1297 (2003). A recent paper showing the intracellular rearrangement of HIV-1 towards the immunological synapse.
Patterson, S., Rae, A., Hockey, N., Gilmour, J. & Gotch, F. Plasmacytoid dendritic cells are highly susceptible to human immunodeficiency virus type 1 infection and release infectious virus. J. Virol. 75, 6710–6713 (2001).
Choi, Y. K., Fallert, B. A., Murphey-Corb, M. A. & Reinhart, T. A. Simian immunodeficiency virus dramatically alters expression of homeostatic chemokines and dendritic cell markers during infection in vivo. Blood 101, 1684–1691 (2003).
Fong, L., Mengozzi, M., Abbey, N. W., Herndier, B. G. & Engleman, E. G. Productive infection of plasmacytoid dendritic cells with human immunodeficiency virus type 1 is triggered by CD40 ligation. J. Virol. 76, 11033–11041 (2002).
Frank, I. et al. Infectious and whole inactivated simian immunodeficiency viruses interact similarly with primate dendritic cells (DCs): differential intracellular fate of virions in mature and immature DCs. J. Virol. 76, 2936–2951 (2002).
Raftery, M. J. et al. Targeting the function of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity 15, 997–1009 (2001).
Andrews, D. M., Andoniou, C. E., Granucci, F., Ricciardi-Castagnoli, P. & Degli-Esposti, M. A. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nature Immunol. 2, 1077–1084 (2001).
Turville, S. G. et al. Diversity of receptors binding HIV on dendritic cell subsets. Nature Immunol. 3, 975–983 (2002). This paper shows that potentially other C-type lectins are also involved in the capture of HIV-1-encoded envelope glycoprotein gp120.
Turville, S. G. et al. HIV gp120 receptors on human dendritic cells. Blood 98, 2482–2488 (2001).
Kavanagh, D. G. & Bhardwaj, N. A division of labor: DC subsets and HIV receptor diversity. Nature Immunol. 3, 891–893 (2002).
Weissman, D. & Fauci, A. S. Role of dendritic cells in immunopathogenesis of human immunodeficiency virus infection. Clin. Microbiol. Rev. 10, 358–367 (1997).
Nguyen, D. G. & Hildreth, J. E. Involvement of macrophage mannose receptor in the binding and transmission of HIV by macrophages. Eur. J. Immunol. 33, 483–493 (2003).
Sanders, R. W. et al. Differential transmission of human immunodeficiency virus type 1 by distinct subsets of effector dendritic cells. J. Virol. 76, 7812–7821 (2002).
Larsson, M. et al. Activation of HIV-1 specific CD4+ and CD8+ T cells by human dendritic cells: roles for cross-presentation and non-infectious HIV-1 virus. AIDS 16, 1319–1329 (2002).
Patel, M. et al. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res. Hum. Retroviruses 9, 167–174 (1993).
Mondor, I., Ugolini, S. & Sattentau, Q. J. Human immunodeficiency virus type 1 attachment to HeLa CD4+ cells is CD4 independent and gp120 dependent and requires cell surface heparans. J. Virol. 72, 3623–3634 (1998).
Saphire, A. C., Bobardt, M. D., Zhang, Z., David, G. & Gallay, P. A. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J. Virol. 75, 9187–9200 (2001).
Callebaut, C., Nisole, S., Briand, J. P., Krust, B. & Hovanessian, A. G. Inhibition of HIV infection by the cytokine midkine. Virology 281, 248–264 (2001).
van Kooyk, Y., Appelmelk, B. & Geijtenbeek, T. B. A fatal attraction: Mycobacterium tuberculosis and HIV-1 target DC-SIGN to escape immune surveillance. Trends Mol. Med. 9, 153–159 (2003).
Nigou, J., Zelle-Rieser, C., Gilleron, M., Thurnher, M. & Puzo, G. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J. Immunol. 166, 7477–7485 (2001).
Soilleux, E. J. et al. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J. Leukoc. Biol. 71, 445–457 (2002).
Kaufmann, S. H. & Schaible, U. E. A dangerous liaison between two major killers: Mycobacterium tuberculosis and HIV target dendritic cells through DC-SIGN. J. Exp. Med. 197, 1–5 (2003).
Nigou, J., Vercellone, A. & Puzo, G. New structural insights into the molecular deciphering of mycobacterial lipoglycan binding to C-type lectins: lipoarabinomannan glycoform characterization and quantification by capillary electrophoresis at the subnanomole level. J. Mol. Biol. 299, 1353–1362 (2000).
Ehlers, M. R. & Daffe, M. Interactions between Mycobacterium tuberculosis and host cells: are mycobacterial sugars the key? Trends Microbiol. 6, 328–335 (1998).
Schlesinger, L. S., Hull, S. R. & Kaufman, T. M. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J. Immunol. 152, 4070–4079 (1994).
Akira, S., Takeda, K. & Kaisho, T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nature Immunol. 2, 675–680 (2001).
Chatterjee, D. & Khoo, K. H. Mycobacterial lipoarabinomannan: an extraordinary lipoheteroglycan with profound physiological effects. Glycobiology 8, 113–120 (1998).
Sada, E., Brennan, P. J., Herrera, T. & Torres, M. Evaluation of lipoarabinomannan for the serological diagnosis of tuberculosis. J. Clin. Microbiol. 28, 2587–2590 (1990).
Tsuji, S. et al. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette–Guerin: involvement of toll-like receptors. Infect. Immun. 68, 6883–6890 (2000).
Jonuleit, H., Schmitt, E., Schuler, G., Knop, J. & Enk, A. H. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 192, 1213–1222 (2000).
Jiao, X. et al. Dendritic cells are host cells for mycobacteria in vivo that trigger innate and acquired immunity. J. Immunol. 168, 1294–1301 (2002).
Fortsch, D., Rollinghoff, M. & Stenger, S. IL-10 converts human dendritic cells into macrophage-like cells with increased antibacterial activity against virulent Mycobacterium tuberculosis. J. Immunol. 165, 978–987 (2000).
Grunebach, F., Weck, M. M., Reichert, J. & Brossart, P. Molecular and functional characterization of human Dectin-1. Exp. Hematol. 30, 1309–1315 (2002).
Brown, G. D. & Gordon, S. Immune recognition. A new receptor for β-glucans. Nature 413, 36–37 (2001).
Brown, G. D. et al. Dectin-1 mediates the biological effects of β-glucans. J. Exp. Med. 197, 1119–1124 (2003). This paper, together with reference 138, shows that C-type lectins can modulate TLR signalling.
Gantner, B. N., Simmons, R. M., Canavera, S. J., Akira, S. & Underhill, D. M. Collaborative induction of inflammatory responses by Dectin-1 and Toll-like receptor 2. J. Exp. Med. 197, 1107–1117 (2003).
Geijtenbeek, T. B. et al. Marginal zone macrophages express a murine homologue of DC-SIGN that captures blood-borne antigens in vivo. Blood 100, 2908–2916 (2002).
Park, C. G. et al. Five mouse homologues of the human dendritic cell C-type lectin, DC-SIGN. Int. Immunol. 13, 1283–1290 (2001).
Acknowledgements
We thank all former and present members of our group, including our collaborators whose work has helped shape the ideas. We thank A. Engering and S. van Vliet for critical reading of the manuscript. We are grateful to the Netherlands Organization for Scientific Research, the AIDS foundation, the Dutch stomach, kidney, liver organization and the Dutch Cancer Foundation for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Kooyk, Y., Geijtenbeek, T. DC-SIGN: escape mechanism for pathogens. Nat Rev Immunol 3, 697–709 (2003). https://doi.org/10.1038/nri1182
Issue Date:
DOI: https://doi.org/10.1038/nri1182
This article is cited by
-
Differentiation, maturation, and collection of THP-1-derived dendritic cells based on a PEG hydrogel culture platform
Biotechnology Letters (2024)
-
Association of CD209 promoter variants and tuberculosis infection susceptibility, AIDS development, and treatment response outcomes among the HIV-1 Moroccan population
The Nucleus (2023)
-
CSF-1-induced DC-SIGN+ macrophages are present in the ovarian endometriosis
Reproductive Biology and Endocrinology (2022)
-
Platelets in pediatric and neonatal sepsis: novel mediators of the inflammatory cascade
Pediatric Research (2022)
-
Carbohydrates from Pseudomonas aeruginosa biofilms interact with immune C-type lectins and interfere with their receptor function
npj Biofilms and Microbiomes (2021)