Key Points
-
The subunits of the immune receptors that participate in signalling have at least one tyrosine-based activation motif in their cytoplasmic domains. Signalling is initiated by the phosphorylation of canonical tyrosine residues that are located in these domains. The phosphorylated forms of the cytoplasmic domains, and other scaffolding proteins, nucleate the association of kinases, phosphatases and adaptors, but the binding is reversible and the structures that form are temporary.
-
We review simple and detailed mathematical models of immune-receptor signalling and what we have learned from them.
-
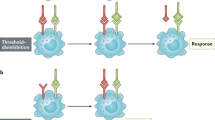
The serial triggering of T-cell receptors has been suggested to explain how a small number of peptide–MHC complexes displayed on antigen-presenting cells can trigger the downregulation of the cell-surface expression of a large number of T-cell receptors.
-
From the binding properties and concentrations of the interacting ligands and receptors, it is straightforward to decide whether serial engagement occurs. However, a model of cell signalling must associate the lifetime of the bound ligand–receptor complex with a cellular response. McKeithan's simple kinetic proofreading model captures an important feature of the signalling cascade — that there is a temporal delay between binding and signalling.
-
Serial engagement requires rapid dissociation. Kinetic proofreading requires receptors to remain bound to ligands for long enough for the appropriate signalling complexes to form. For T-cell activation, competition between serial engagement and kinetic proofreading leads to the prediction that maximal signal generation occurs for an intermediate value of the lifetime of the ligand–receptor bond.
-
Detailed models comprise a limited set of proteins that participate in cell signalling and the interactions that are assumed to occur between them. Unlike simple models, these models can make predictions about the states of the individual components, while providing insights into how the components function together as a system. Although few such models have been developed for immune-receptor signalling, the present examples indicate that these models will become an important part of ongoing efforts to understanding cell signalling at the molecular level.
-
One impediment to creating and understanding detailed models is the problem of combinatorial complexity: because components can be modified and combine in many ways, the number of possible complexes that can form during a signalling cascade is exceedingly large. As a result, signalling cascades are large temporal biochemical networks and not simple chemical pathways.
Abstract
The process of signalling through receptors of the immune system involves highly connected networks of interacting components. Understanding the often counter-intuitive behaviour of these networks requires the development of mathematical and computational models. Here, we focus on the application of these models to understand signalling through immune receptors that are involved in antigen recognition. Simple models, which ignore the details of the signalling machinery, have provided considerable insight into how ligand–receptor binding properties affect signalling outcomes. Detailed models, which include specific molecular components and interactions beyond the ligand and receptor, are difficult to develop but have already provided new mechanistic understanding and uncovered relationships that are difficult to detect by experimental observation alone. They offer hope that models might eventually predict the full spectrum of signalling behaviour.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Keegan, A. D. & Paul, W. E. Multichain immune recognition receptors: similarities in structure and signaling pathways. Immunol. Today 13, 63–68 (1992).
Reth, M. Antigen receptor tail clue. Nature 338, 383–384 (1989).
Cambier, J. C. Antigen and Fc receptor signaling: the awesome power of the immunoreceptor tyrosine-based activation motif (ITAM). J. Immunol. 155, 3281–3285 (1995).
Daeron, M. Fc receptor biology. Annu. Rev. Immunol. 15, 203–234 (1997).
Boniface, J. J. et al. Initiation of signal transduction through the T cell receptor requires the multivalent engagement of peptide/MHC ligands. Immunity 9, 459–466 (1998).
Cochran, J., Cameron, T. & Stern, L. The relationship of MHC–peptide binding and T cell activation probed using chemically defined MHC class II oligomers. Immunity 12, 241–250 (2000).
Metzger, H. Transmembrane signaling: the joy of aggregation. J. Immunol. 149, 1477–1487 (1992).
Delisi, C. The magnitude of signal amplification by ligand-induced receptor clustering. Nature 289, 322–323 (1981).
Irving, B. A., Chan, A. C. & Weiss, A. Functional characterization of a signal transducing motif present in the T cell antigen receptor ζ chain. J. Exp. Med. 177, 1093–1103 (1993).
Wange, R. L., Malek, S. N., Desiderio, S. & Samelson, L. E. Tandem SH2 domains of ZAP-70 bind to TCRζ and CD3ε from activated Jurkat T cells. J. Biol. Chem. 268, 19797–19801 (1993).
Kihara, H. & Siraganian, R. P. Src homology-2 domains of Syk and Lyn bind to tyrosine-phosphorylated subunits of the high-affinity IgE receptor. J. Biol. Chem. 269, 22427–22432 (1994).
Bu, J. -Y., Shaw, A. S. & Chan, A. C. Analysis of the interaction of Zap-70 and Syk protein-tyrosine kinases with the T-cell antigen receptor by plasmon resonance. Proc. Natl Acad. Sci. USA 92, 5106–5110 (1995).
Kumaran, S., Grucza, R. A. & Waksman, G. The tandem Src homology 2 domain of the Syk kinase: a molecular device that adapts to interphosphotyrosine distances. Proc. Natl Acad. Sci. USA 100, 14828–14833 (2003).
Bunnell, S. C. et al. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J. Cell Biol. 158, 1263–1275 (2002).
Torigoe, C., Inman, J. K. & Metzger, H. An unusual mechanism for ligand antagonism. Science 281, 568–572 (1998). Showed that the FcεRI-initiated signalling cascade is subject to kinetic proofreading, and proposed a mechanism for ligand antagonism, in which antagonists bind receptors and sequester the crucial initiating kinase, LYN.
Dittel, B. N., Stefanova, I., Germain, R. N. & Janeway, C. A. Cross-antagonism of a T cell clone expressing two distinct T cell receptors. Immunity 11, 289–298 (1999).
Robertson, J. M. & Evavold, B. D. Cutting edge: Dueling TCRs: peptide antagonism of CD4+ T cells with dual antigen specificities. J. Immunol. 163, 1750–1754 (1999).
Stefanova, I. et al. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nature Immunol. 4, 248–254 (2003).
Wofsy, C., Torigoe, C., Kent, U. M., Metzger, H. & Goldstein, B. Exploiting the difference between intrinsic and extrinsic kinases: implications for regulation of signaling by immunoreceptors. J. Immunol. 159, 5984–5992 (1997).
Burack, W. R. & Shaw, A. S. Signal transduction: hanging on a scaffold. Curr. Opin. Cell Biol. 12, 211–216 (2000).
Levchenko, A., Bruck, J. & Sternberg, P. W. Scaffold proteins may biphasically affect the levels of mitogen-activated protein kinase signaling and reduce its threshold properties. Proc. Natl Acad. Sci. USA 97, 5818–5823 (2000).
Faeder, J. R. et al. Investigation of early events in FcεRI-mediated signaling using a detailed mathematical model. J. Immunol. 170, 3769–3781 (2003). Presented a detailed model of the network of early phosphorylation events that occur during signalling through FcεRI and lead to SYK activation, and used this model to analyse various data on the phosphorylation of the β- and γ-receptor subunit ITAMs.
Markevich, N. I., Hoek, J. B. & Kholodenko, B. N. Signaling switches and bistability arising from multisite phosphorylation in protein kinase cascades. J. Cell Biol. 164, 353–359 (2004).
Simons, K. & Ikonen, E. Functional rafts in cell membranes. Nature 387, 569–572 (1997).
Qi, S. Y., Groves, J. T. & Chakraborty, A. K. Synaptic pattern formation during cellular recognition. Proc. Natl Acad. Sci. USA 98, 6548–6553 (2001). Used a spatial-kinetic model of immunological-synapse formation to show that receptor proteins at the synapse have a strong tendency to self-organize in the presence of a stimulating ligand, which might be reinforced by active cellular processes but does not require them.
Burroughs, N. J. & Wulfing, C. Differential segregation in a cell–cell contact interface: the dynamics of the immunological synapse. Biophys. J. 83, 1784–1796 (2002).
Coombs, D., Kalergis, A. M., Nathenson, S. G., Wofsy, C. & Goldstein, B. Activated TCRs remain marked for internalization after dissociation from pMHC. Nature Immunol. 3, 926–931 (2002).
Lee, K. H. et al. The immunological synapse balances T cell receptor signaling and degradation. Science 302, 1218–1222 (2003). Combined modelling and experimental study of the role of the cSMAC in T-cell activation. Showed that enhanced TCR signalling occurs at the cSMAC initially, but later it becomes the site of rapid TCR degradation and diminution of signalling.
Valitutti, S., Muller, S., Cella, M., Padovan, E. & Lanzavecchia, A. Serial triggering of many T-cell receptors by a few peptide–MHC complexes. Nature 375, 148–151 (1995). Introduced the idea of serial triggering of TCRs. This paper and reference 31 present data showing that when there are only a few cognate peptide–MHC complexes on an APC, a single peptide–MHC complex can trigger the downregulation of numerous TCRs.
Valitutti, S. & Lanzavecchia, A. Serial triggering of TCRs: a basis for the sensitivity and specificity of antigen recognition. Immunol. Today 18, 299–304 (1997).
Itoh, Y., Hemmer, B., Martin, R. & Germain, R. N. Serial TCR engagement and down-modulation by peptide–MHC molecule ligands: relationship to the quality of individual TCR signaling events. J. Immunol. 162, 2073–2080 (1999).
San José, E., Borroto, A., Niedergang, F., Alcover, A. & Alarcon, B. Triggering the TCR complex causes the downregulation of nonengaged receptors by a signal transduction-dependent mechanism. Immunity 12, 161–170 (2000).
Wofsy, C., Coombs, D. & Goldstein, B. Calculations show substantial serial engagement of T cell receptors. Biophys. J. 80, 606–612 (2001).
Hlavacek, W. S., Percus, J. K., Percus, O. E., Perelson, A. S. & Wofsy, C. Retention of antigen on follicular dendritic cells and B lymphocytes through complement-mediated multivalent ligand–receptor interactions: theory and application to HIV treatment. Math. Biosci. 176, 185–202 (2002).
McKeithan, T. W. Kinetic proofreading in T-cell receptor signal-transduction. Proc. Natl Acad. Sci. USA 92, 5042–5046 (1995). Introduced the idea of kinetic proofreading in the context of receptor signalling and presented a mathematical model of kinetic proofreading to explain how T cells can discriminate between ligands with moderately different dissociation rates.
Matsui, K., Boniface, J. J., Steffner, P., Reay, P. A. & Davis, M. M. Kinetics of T-cell receptor binding to peptide/I–Ek complexes: correlation of the dissociation rate with T-cell responsiveness. Proc. Natl Acad. Sci. USA 91, 12862–12866 (1994).
Lyons, D. S. et al. A TCR binds to antagonist ligands with lower affinities and faster dissociation rates than to agonists. Immunity 5, 53–61 (1996).
Kersh, G. J., Kersh, E. N., Fremont, D. H. & Allen, P. M. High- and low-potency ligands with similar affinities for the TCR: the importance of kinetics in TCR signaling. Immunity 9, 817–826 (1998).
Ding, Y. H., Baker, B. M., Garboczi, D. N., Biddison, W. E. & Wiley, D. C. Four A6-TCR/peptide/HLA-A2 structures that generate very different T cell signals are nearly identical. Immunity 11, 45–56 (1999).
Holler, P. D. & Kranz, D. M. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity 18, 255–264 (2003).
Sykulev, Y., Vugmeyster, Y., Brunmark, A., Ploegh, H. L. & Eisen, H. N. Peptide antagonism and T cell receptor interactions with peptide–MHC complexes. Immunity 9, 475–483 (1998).
Alam, S. M. et al. T-cell-receptor affinity and thymocyte positive selection. Nature 381, 616–620 (1996).
Baker, B. M., Gagnon, S. J., Biddison, W. E. & Wiley, D. C. Conversion of a T cell antagonist into an agonist by repairing a defect in the TCR/peptide/MHC interface: implications for TCR signaling. Immunity 13, 475–484 (2000).
Krogsgaard, M. et al. Evidence that structural rearrangements and/or flexibility during TCR binding can contribute to T cell activation. Mol. Cell 12, 1367–1378 (2003).
Kersh, E. N., Shaw, A. S. & Allen, P. M. Fidelity of T cell activation through multistep T cell receptor ζ phosphorylation. Science 281, 572–575 (1998).
Rabinowitz, J. D., Beeson, C., Lyons, D. S., Davis, M. M. & McConnell, H. M. Kinetic discrimination in T-cell activation. Proc. Natl Acad. Sci. USA 93, 1401–1405 (1996).
Lord, G. M., Lechler, R. I. & George, A. J. T. A kinetic differentiation model for the action of altered TCR ligands. Immunol. Today 20, 33–39 (1999).
Chan, C., George, A. J. T. & Stark, J. T cell sensitivity and specificity — kinetic proofreading revisited. Discrete Contin. Dyn. Syst. Ser. B 3, 343–360 (2003).
Chan, C., George, A. J. T. & Stark, J. Cooperative enhancement of specificity in a lattice of T cell receptors. Proc. Natl Acad. Sci. USA 98, 5758–5763 (2001).
Liu, Z. J., Haleem-Smith, H., Chen, H. X. & Metzger, H. Unexpected signals in a system subject to kinetic proofreading. Proc. Natl Acad. Sci. USA 98, 7289–7294 (2001). Showed that many FcεRI-mediated mast-cell responses follow kinetic proofreading, as described in reference 15, but also found an exception — a rapidly dissociating ligand that activated a late response but failed to activate earlier responses, similar to the findings for T cells in reference 54.
Eglite, S., Morin, J. M. & Metzger, H. Synthesis and secretion of monocyte chemotactic protein-1 stimulated by the high affinity receptor for IgE. J. Immunol. 170, 2680–2687 (2003).
Hlavacek, W. S., Redondo, A., Metzger, H., Wofsy, C. & Goldstein, B. Kinetic proofreading models for cell signaling predict ways to escape kinetic proofreading. Proc. Natl Acad. Sci. USA 98, 7295–7300 (2001). Extended McKeithan's kinetic proofreading model to aggregating receptor systems and to receptor systems in which signalling depends on an external initiating kinase. Considered the kinetics of signalling molecules that are generated by aggregated receptors but do not remain associated with the receptor complex.
Hlavacek, W. S., Redondo, A., Wofsy, C. & Goldstein, B. Kinetic proofreading in receptor-mediated transduction of cellular signals: receptor aggregation, partially activated receptors, and cytosolic messengers. Bull. Math. Biol. 64, 887–911 (2002).
Rosette, C. et al. The impact of duration versus extent of TCR occupancy on T cell activation: a revision of the kinetic proofreading model. Immunity 15, 59–70 (2001). Showed that a low-affinity tetrameric peptide–MHC complex generated a late T-cell response but not early responses. Proposed that T-cell responses are triggered by a cumulative signal.
Faroudi, M., Zaru, R., Paulet, P., Muller, S. & Valitutti, S. Cutting edge: T lymphocyte activation by repeated immunological synapse formation and intermittent signaling. J. Immunol. 171, 1128–1132 (2003).
Muller, S., Demotz, S., Bulliard, C. & Valitutti, S. Kinetics and extent of protein tyrosine kinase activation in individual T cells upon antigenic stimulation. Immunology 97, 287–293 (1999).
Borovsky, Z., Mishan-Eisenberg, G., Yaniv, E. & Rachmilewitz, J. Serial triggering of T cell receptors results in incremental accumulation of signaling intermediates. J. Biol. Chem. 277, 21529–21536 (2002).
Rachmilewitz, J. & Lanzavecchia, A. A temporal and spatial summation model for T-cell activation: signal integration and antigen decoding. Trends Immunol. 23, 592–595 (2002).
Sykulev, Y., Joo, M., Vturina, I., Tsomides, T. J. & Eisen, H. N. Evidence that a single peptide–MHC complex on a target cell can elicit a cytolytic T cell response. Immunity 4, 565–571 (1996).
Irvine, D. J., Purbhoo, M. A., Krogsgaard, M. & Davis, M. M. Direct observation of ligand recognition by T cells. Nature 419, 845–849 (2002).
Lanzavecchia, A., Iezzi, G. & Viola, A. From TCR engagement to T cell activation: a kinetic view of T cell behavior. Cell 96, 1–4 (1999).
Van Den Berg, H. A., Rand, D. A. & Burroughs, N. J. A reliable and safe T cell repertoire based on low-affinity T cell receptors. J. Theor. Biol. 209, 465–486 (2001).
Kalergis, A. M. et al. Efficient T cell activation requires an optimal dwell-time of interaction between the TCR and the pMHC complex. Nature Immunol. 2, 229–234 (2001). Showed that TCR activation increases to a maximum value and then declines as a function of dwell-time, as predicted by competition between serial engagement and kinetic proofreading.
Sousa, J. & Carneiro, J. A mathematical analysis of TCR serial triggering and down-regulation. Eur. J. Immunol. 30, 3219–3227 (2000).
Goldstein, B. et al. Modeling the early signaling events mediated by FcεRI. Mol. Immunol. 38, 1213–1219 (2002).
Hlavacek, W. S., Faeder, J. R., Blinov, M. L., Perelson, A. S. & Goldstein, B. The complexity of complexes in signal transduction. Biotechnol. Bioeng. 84, 783–794 (2003).
Wofsy, C., Kent, U. M., Mao, S. Y., Metzger, H. & Goldstein, B. Kinetics of tyrosine phosphorylation when IgE dimers bind to Fcε receptors on rat basophilic leukemia cells. J. Biol. Chem. 270, 20264–20272 (1995).
Torigoe, C., Goldstein, B., Wofsy, C. & Metzger, H. Shuttling of initiating kinase between discrete aggregates of the high affinity receptor for IgE regulates the cellular response. Proc. Natl Acad. Sci. USA 94, 1372–1377 (1997).
Wofsy, C., Vonakis, B. M., Metzger, H. & Goldstein, B. One Lyn molecule is sufficient to initiate phosphorylation of aggregated high-affinity IgE receptors. Proc. Natl Acad. Sci. USA 96, 8615–8620 (1999).
Young, R. M., Holowka, D. & Baird, B. A lipid raft environment enhances Lyn kinase activity by protecting the active site tyrosine from dephosphorylation. J. Biol. Chem. 278, 20746–20752 (2003).
Rafnar, T. et al. Stimulation of the high-affinity IgE receptor results in the tyrosine phosphorylation of a 60 kD protein which is associated with the protein-tyrosine kinase Csk. Mol. Immunol. 35, 249–257 (1998).
Ohtake, H., Ichikawa, N., Okada, M. & Yamashita, T. Cutting edge: Transmembrane phosphoprotein Csk-binding protein/phosphoprotein associated with glycosphingolipid-enriched microdomains as a negative feedback regulator of mast cell signaling through the FcεRI. J. Immunol. 168, 2087–2090 (2002).
Siraganian, R. P. Mast cell signal transduction from the high-affinity IgE receptor. Curr. Opin. Immunol. 15, 639–646 (2003).
Mao, S. Y. & Metzger, H. Characterization of protein-tyrosine phosphatases that dephosphorylate the high affinity IgE receptor. J. Biol. Chem. 272, 14067–14073 (1997).
Lee, S. J., Hori, Y., Groves, J. T., Dustin, M. L. & Chakraborty, A. K. The synapse assembly model. Trends Immunol. 23, 500–502 (2002).
Coombs, D., Dembo, M., Wofsy, C. & Goldstein, B. Equilibrium thermodynamics of cell–cell adhesion mediated by multiple ligand–receptor pairs. Biophys. J. 86, 1408–1423 (2004).
Chakraborty, A. K., Dustin, M. L. & Shaw, A. S. In silico models for cellular and molecular immunology: successes, promises and challenges. Nature Immunol. 4, 933–936 (2003).
Dustin, M. L. et al. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell 94, 667–677 (1998).
Hopfield, J. J. Kinetic proofreading: a new mechanism for reducing errors in biosynthetic processes requiring high specificity. Proc. Natl Acad. Sci. USA 71, 4135–4139 (1974).
Gillespie, D. T. A general method for numerically simulating the stochastic time evolution of coupled chemical reactions. J. Comp. Phys. 22, 403–434 (1976).
Gibson, M. A. & Bruck, J. Efficient exact stochastic simulation of chemical systems with many species and many channels. J. Phys. Chem. A 104, 1876–1889 (2000).
Slepchenko, B. M., Schaff, J. C., Macara, I. & Loew, L. M. Quantitative cell biology with the virtual cell. Trends Cell Biol. 13, 570–576 (2003).
Pleiman, C. M. et al. Distinct p53/56lyn and p59fyn domains associate with nonphosphorylated and phosphorylated Ig-α. Proc. Natl Acad. Sci. USA 91, 4268–4272 (1994).
Vonakis, B. M., Chen, H. X., Haleemsmith, H. & Metzger, H. The unique domain as the site on Lyn kinase for its constitutive association with the high affinity receptor for IgE. J. Biol. Chem. 272, 24072–24080 (1997).
Turner, H. & Kinet, J. P. Signalling through the high-affinity IgE receptor FcεRI. Nature 402, B24–B30 (1999).
Kurosaki, T. Molecular mechanisms in B cell antigen receptor signaling. Curr. Opin. Immunol. 9, 309–318 (1997).
Kane, L. P., Lin, J. & Weiss, A. Signal transduction by the TCR for antigen. Curr. Opin. Immunol. 12, 242–249 (2000).
Davis, S. J. et al. The nature of molecular recognition by T cells. Nature Immunol. 4, 217–224 (2003).
Acknowledgements
We thank the anonymous reviewers for constructive comments. This work was supported by grants from the National Institutes of Health and the US Department of Energy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- MULTICHAIN IMMUNE-RECOGNITION RECEPTOR
-
(MIRR). The prototypical members of the MIRR family are the B-cell receptor, the T-cell receptor and the high-affinity IgE receptor (FcεRI). Each of these cell-surface receptors is multimeric and involved in antigen recognition.
- SRC HOMOLOGY 2 DOMAINS
-
(SH2 domains). Protein domains that bind phosphorylated tyrosine residues and are present in many signalling proteins, including the kinases of the SRC and SYK (spleen tyrosine kinase) families.
- RATE CONSTANTS
-
Parameters with a constant value in a mathematical expression for the rate of a chemical reaction. The rate of the elementary chemical reaction A→B is given by k[A], where k is the rate constant and [A] is the concentration of species A.
- PARTIAL DIFFERENTIAL EQUATIONS
-
(PDEs). Differential equations that involve more than one independent variable. Often the independent variables of interest are time and position in space.
- ORDINARY DIFFERENTIAL EQUATIONS
-
(ODEs). Differential equations that involve only one independent variable, such as d[A]/dt = −k[A], where the independent variable is time (t), and the concentration of the species [A] is a dependent variable that depends on t. k is the rate constant. A system of ODEs involves multiple dependent variables, all of which are functions of the same independent variable.
- LIPID RAFTS
-
Lipid rafts are microdomains of the cell membrane that are enriched in sphingolipids. Several membrane-associated signalling molecules, such as LYN, are concentrated in these rafts.
- IMMUNOLOGICAL SYNAPSE
-
A stable region of contact between a T cell and an antigen-presenting cell that forms through cell–cell interaction of adhesion molecules. The mature immunological synapse contains two distinct, stable membrane domains: a central cluster of TCRs, known as the central supramolecular activation cluster (cSMAC) and a surrounding ring of adhesion molecules known as the peripheral supramolecular activation cluster (pSMAC).
Rights and permissions
About this article
Cite this article
Goldstein, B., Faeder, J. & Hlavacek, W. Mathematical and computational models of immune-receptor signalling. Nat Rev Immunol 4, 445–456 (2004). https://doi.org/10.1038/nri1374
Issue Date:
DOI: https://doi.org/10.1038/nri1374
This article is cited by
-
BioNetGMMFit: estimating parameters of a BioNetGen model from time-stamped snapshots of single cells
npj Systems Biology and Applications (2023)
-
Engineering AvidCARs for combinatorial antigen recognition and reversible control of CAR function
Nature Communications (2020)
-
Assessing the interactions between radiotherapy and antitumour immunity
Nature Reviews Clinical Oncology (2019)
-
A review of inflammatory mechanism in airway diseases
Inflammation Research (2019)
-
Biological Implications of Dynamical Phases in Non-equilibrium Networks
Journal of Statistical Physics (2016)