Key Points
-
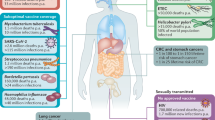
Sexually transmitted viruses (STVs) are a major cause of morbidity and mortality in humans worldwide. Yet, for the most part, vaccines that prevent transmission of STVs are not available.
-
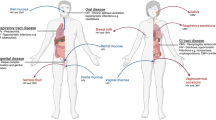
Certain STVs, such as herpes simplex virus type 2 and human papillomavirus, cause much more severe disease in women than in men. One of the underlying reasons for this variation is the differences in the anatomy of the genital mucosa between females and males; the female genital tract has a much larger area for viral invasion and contains many more target cell types.
-
Innate immunity in the genital mucosa is provided by a physical barrier (such as mucus and epithelial cells), antimicrobial factors (such as antimicrobial peptides, lactoferrin and complement) and endogenous microflora. In addition, innate immune cells survey the environment for pathogen invasion by expression of a wide range of pattern recognition receptors.
-
Adaptive immunity in the genital mucosa is mediated by antibodies (IgG in vaginal transudate and IgA from the cervix), CD4+ T cells and CD8+ T cells. After viral clearance, foci of memory lymphocytes form near the vaginal epithelium, which provides an immediate source of virus-specific T and B cells, should a secondary infection occur.
-
Immune correlates of protection for each STV must be understood before a successful vaccine can be developed. Vaccines against HIV-1 must establish a robust local memory CD8+ T cell population without recruiting memory CD4+ T cells that can become targets for viral replication.
Abstract
Mucosal surfaces are exploited as a portal of entry into hosts by a wide variety of microorganisms. Over the past decade, an advanced understanding of the immune system of the gastrointestinal and the respiratory mucosae has been gained. However, despite the fact that many viruses are transmitted sexually through the genital tract, the immune system of the male and female genital mucosae has received much less attention. Here, I describe and highlight differences in the innate and adaptive immune systems of the genital and intestinal mucosae, and discuss some of the challenges we face in the development of successful vaccines against sexually transmitted viral pathogens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Starnbach, M. N. & Roan, N. R. Conquering sexually transmitted diseases. Nature Rev. Immunol. 8, 313–317 (2008).
Joint United Nations Programme on HIV/Acquired Immune Deficiency Syndrome/World Health Organization. AIDS epidemic update 2007 (UNAIDS/WHO, Geneva, 2007).
Joint United Nations Programme on HIV/Acquired Immune Deficiency Syndrome/World Health Organization. Report on the global AIDS epidemic (UNAIDS/WHO, Geneva, 2008).
Lafferty, W. E. The changing epidemiology of HSV-1 and HSV-2 and implications for serological testing. Herpes 9, 51–55 (2002).
Frazer, I. H. Prevention of cervical cancer through papillomavirus vaccination. Nature Rev. Immunol. 4, 46–54 (2004).
World Health Organization. Comprehensive cervical cancer control: a guide to essential practice (WHO, Geneva, 2006).
Wira, C. R., Fahey, J. V., Sentman, C. L., Pioli, P. A. & Shen, L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol. Rev. 206, 306–335 (2005).
Tobian, A. A., Gray, R. H. & Quinn, T. C. Male circumcision for the prevention of acquisition and transmission of sexually transmitted infections: the case for neonatal circumcision. Arch. Pediatr. Adolesc. Med. 164, 78–84 (2010).
Iwasaki, A. Mucosal dendritic cells. Annu. Rev. Immunol. 25, 381–418 (2007).
Fussell, E. N., Kaack, M. B., Cherry, R. & Roberts, J. A. Adherence of bacteria to human foreskins. J. Urol. 140, 997–1001 (1988).
Naylor, S. W. et al. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 71, 1505–1512 (2003).
Hladik, F. & McElrath, M. J. Setting the stage: host invasion by HIV. Nature Rev. Immunol. 8, 447–457 (2008).
Shattock, R. J. & Moore, J. P. Inhibiting sexual transmission of HIV-1 infection. Nature Rev. Microbiol. 1, 25–34 (2003).
Haase, A. T. Targeting early infection to prevent HIV-1 mucosal transmission. Nature 464, 217–223 (2010).
Keele, B. F. et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl Acad. Sci. USA 105, 7552–7557 (2008).
Abrahams, M. R. et al. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-poisson distribution of transmitted variants. J. Virol. 83, 3556–3567 (2009). References 15 and 16 show that a single HIV-1 virus is responsible for establishing the majority of clinical infection in humans.
Spear, P. G., Eisenberg, R. J. & Cohen, G. H. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275, 1–8 (2000).
Koelle, D. M. & Corey, L. Herpes simplex: insights on pathogenesis and possible vaccines. Annu. Rev. Med. 59, 381–395 (2008).
Hernandez, B. Y. et al. Circumcision and human papillomavirus infection in men: a site-specific comparison. J. Infect. Dis. 197, 787–794 (2008).
Kines, R. C., Thompson, C. D., Lowy, D. R., Schiller, J. T. & Day, P. M. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc. Natl Acad. Sci. USA 106, 20458–20463 (2009). An intriguing report that shows that HPV evolved a two-phase mechanism of entry; initial steps occur on the basement membrane followed by capsid transfer to basal epithelial cells.
Valore, E. V., Park, C. H., Igreti, S. L. & Ganz, T. Antimicrobial components of vaginal fluid. Am. J. Obstet. Gynecol. 187, 561–568 (2002).
Hooper, L. V. & Macpherson, A. J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nature Rev. Immunol. 10, 159–169 (2010).
Hooper, L. V. & Gordon, J. I. Commensal host-bacterial relationships in the gut. Science 292, 1115–1118 (2001).
Dethlefsen, L., McFall-Ngai, M. & Relman, D. A. An ecological and evolutionary perspective on human–microbe mutualism and disease. Nature 449, 811–818 (2007).
Qin, J. et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 (2010).
Fredricks, D. N., Fiedler, T. L. & Marrazzo, J. M. Molecular identification of bacteria associated with bacterial vaginosis. N. Engl. J. Med. 353, 1899–1911 (2005).
Cutler, B. & Justman, J. Vaginal microbicides and the prevention of HIV transmission. Lancet Infect. Dis. 8, 685–697 (2008).
Kim, T. K. et al. Heterogeneity of vaginal microbial communities within individuals. J. Clin. Microbiol. 47, 1181–1189 (2009).
Milligan, G. N. Neutrophils aid in protection of the vaginal mucosae of immune mice against challenge with herpes simplex virus type 2. J. Virol. 73, 6380–6386 (1999).
Orange, J. S. Human natural killer cell deficiencies. Curr. Opin. Allergy Clin. Immunol. 6, 399–409 (2006).
Nishimura, H. et al. Intraepithelial γδ T cells may bridge a gap between innate immunity and acquired immunity to herpes simplex virus type 2. J. Virol. 78, 4927–4930 (2004).
Cohen, G. B. et al. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10, 661–671 (1999).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Sadler, A. J. & Williams, B. R. Interferon-inducible antiviral effectors. Nature Rev. Immunol. 8, 559–568 (2008).
Iwasaki, A. & Medzhitov, R. Regulation of adaptive immunity by the innate immune system. Science 327, 291–295 (2010).
Iwasaki, A. & Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nature Immunol. 5, 987–995 (2004).
Flacher, V. et al. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J. Immunol. 177, 7959–7967 (2006).
Pichlmair, A. & Reis e Sousa, C. Innate recognition of viruses. Immunity 27, 370–383 (2007).
Chiu, Y. H., Macmillan, J. B. & Chen, Z. J. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138, 576–591 (2009).
Ablasser, A. et al. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nature Immunol. 10, 1065–1072 (2009). References 39 and 40 show that certain classes of dsDNA in the cytoplasm are transcribed by RNA polymerase III, generating ligands for RIG-I for innate recognition.
Martinon, F., Mayor, A. & Tschopp, J. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27, 229–265 (2009).
Kummer, J. A. et al. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J. Histochem. Cytochem. 55, 443–452 (2007).
Burckstummer, T. et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nature Immunol. 10, 266–272 (2009).
Fernandes-Alnemri, T., Yu, J. W., Datta, P., Wu, J. & Alnemri, E. S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 (2009).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Roberts, T. L. et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323, 1057–1060 (2009).
Lund, J., Sato, A., Akira, S., Medzhitov, R. & Iwasaki, A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198, 513–520 (2003).
Krug, A. et al. Herpes simplex virus type 1 activates murine natural interferon-producing cells through Toll-like receptor 9. Blood 103, 1433–1437 (2004).
Kurt-Jones, E. A. et al. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl Acad. Sci. USA 101, 1315–1320 (2004).
Muruve, D. A. et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452, 103–107 (2008).
Rathinam, V. A. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nature Immunol. 11, 395–402 (2010).
Sato, A. & Iwasaki, A. Induction of antiviral immunity requires Toll-like receptor signaling in both stromal and dendritic cell compartments. Proc. Natl Acad. Sci. USA 101, 16274–16279 (2004).
Fujioka, N. et al. Interleukin-18 protects mice against acute herpes simplex virus type 1 infection. J. Virol. 73, 2401–2409 (1999).
Lund, J. M., Linehan, M. M., Iijima, N. & Iwasaki, A. Cutting edge: plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J. Immunol. 177, 7510–7514 (2006).
Yang, R. et al. Papillomavirus-like particles stimulate murine bone marrow-derived dendritic cells to produce α interferon and Th1 immune responses via MyD88. J. Virol. 78, 11152–11160 (2004).
Wolf, D. & Goff, S. P. Host restriction factors blocking retroviral replication. Annu. Rev. Genet. 42, 143–163 (2008).
Browne, E. P. & Littman, D. R. Myd88 is required for an antibody response to retroviral infection. PLoS Pathog. 5, e1000298 (2009). This paper shows that DCs and MyD88 are needed to mount antibody responses and antiviral defence against a mouse retrovirus in vivo .
Beignon, A. S. et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor- viral RNA interactions. J. Clin. Invest. 115, 3265–3275 (2005).
Fitzgerald-Bocarsly, P. & Jacobs, E. S. Plasmacytoid dendritic cells in HIV infection: striking a delicate balance. J. Leukoc. Biol. 87, 609–620 (2010).
Doehle, B. P., Hladik, F., McNevin, J. P., McElrath, M. J. & Gale, M. Jr. Human immunodeficiency virus type 1 mediates global disruption of innate antiviral signaling and immune defenses within infected cells. J. Virol. 83, 10395–10405 (2009).
Medzhitov, R. & Littman, D. HIV immunology needs a new direction. Nature 455, 591 (2008).
Leib, D. A. Counteraction of interferon-induced antiviral responses by herpes simplex viruses. Curr. Top. Microbiol. Immunol. 269, 171–185 (2002).
Lee, H. K. et al. Differential roles of migratory and resident DCs in T cell priming after mucosal or skin HSV-1 infection. J. Exp. Med. 206, 359–370 (2009).
Zhao, X. et al. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J. Exp. Med. 197, 153–162 (2003).
Iijima, N., Linehan, M. M., Saeland, S. & Iwasaki, A. Vaginal epithelial dendritic cells renew from bone marrow precursors. Proc. Natl Acad. Sci. USA 104, 19061–19066 (2007).
Hervouet, C. et al. Langerhans cells prime IL-17-producing T cells and dampen genital cytotoxic responses following mucosal immunization. J. Immunol. 184, 4842–4851 (2010).
Divito, S., Cherpes, T. L. & Hendricks, R. L. A triple entente: virus, neurons, and CD8+ T cells maintain HSV-1 latency. Immunol. Res. 36, 119–126 (2006).
Stanberry, L. R. et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 347, 1652–1661 (2002). Two double-blind, randomized trials of an HSV-2 glycoprotein-D-subunit vaccine showed that the vaccine was only effective against genital herpes in women who were seronegative for both HSV-1 and HSV-2. The vaccine had no efficacy in men, regardless of their HSV serological status.
Matthews, K. et al. Depletion of Langerhans cells in human papillomavirus type 16-infected skin is associated with E6-mediated down regulation of E-cadherin. J. Virol. 77, 8378–8385 (2003).
Ashrafi, G. H., Haghshenas, M., Marchetti, B. & Campo, M. S. E5 protein of human papillomavirus 16 downregulates HLA class I and interacts with the heavy chain via its first hydrophobic domain. Int. J. Cancer 119, 2105–2112 (2006).
Antonsson, A., Payne, E., Hengst, K. & McMillan, N. A. The human papillomavirus type 16 E7 protein binds human interferon regulatory factor-9 via a novel PEST domain required for transformation. J. Interferon Cytokine Res. 26, 455–461 (2006).
Turville, S. G. et al. Diversity of receptors binding HIV on dendritic cell subsets. Nature Immunol. 3, 975–983 (2002).
Hladik, F. et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 26, 257–270 (2007). An excellent analysis of the initial events that occur following HIV-1 entry through the use of ex vivo vaginal organ culture, which shows direct viral infection of CD4+ T cells in the epithelial layer.
McMichael, A. J., Borrow, P., Tomaras, G. D., Goonetilleke, N. & Haynes, B. F. The immune response during acute HIV-1 infection: clues for vaccine development. Nature Rev. Immunol. 10, 11–23 (2010).
Altfeld, M. et al. HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus. Nature 420, 434–439 (2002).
Brandtzaeg, P. Mucosal immunity in the female genital tract. J. Reprod. Immunol. 36, 23–50 (1997).
Moldoveanu, Z., Huang, W. Q., Kulhavy, R., Pate, M. S. & Mestecky, J. Human male genital tract secretions: both mucosal and systemic immune compartments contribute to the humoral immunity. J. Immunol. 175, 4127–4136 (2005).
Bennett, S. R. et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393, 478–480 (1998).
Ridge, J. P., Di Rosa, F. & Matzinger, P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393, 474–478 (1998).
Schoenberger, S. P., Toes, R. E., van der Voort, E. I., Offringa, R. & Melief, C. J. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature 393, 480–483 (1998).
Jennings, S. R., Bonneau, R. H., Smith, P. M., Wolcott, R. M. & Chervenak, R. CD4+ T lymphocytes are required for the generation of the primary but not the secondary CD8+ cytolytic T lymphocyte response to herpes simplex virus in C57BL/6 mice. Cell. Immunol. 133, 234–252 (1991).
Janssen, E. M. et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421, 852–856 (2003).
Shedlock, D. J. & Shen, H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300, 337–339 (2003).
Sun, J. C. & Bevan, M. J. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300, 339–342 (2003).
Nakanishi, Y., Lu, B., Gerard, C. & Iwasaki, A. CD8+ T lymphocyte mobilization to virus-infected tissue requires CD4+ T-cell help. Nature 462, 510–513 (2009).
Iijima, N. et al. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J. Exp. Med. 205, 3041–3052 (2008).
Blaney, J. E. Jr et al. Immunization with a single major histocompatibility complex class I-restricted cytotoxic T-lymphocyte recognition epitope of herpes simplex virus type 2 confers protective immunity. J. Virol. 72, 9567–9574 (1998).
Orr, M. T., Orgun, N. N., Wilson, C. B. & Way, S. S. Cutting edge: recombinant Listeria monocytogenes expressing a single immune-dominant peptide confers protective immunity to herpes simplex virus-1 infection. J. Immunol. 178, 4731–4735 (2007).
Knickelbein, J. E. et al. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science 322, 268–271 (2008). This study shows that CTLs block HSV-1 reactivation by releasing lytic granules into neurons and degrading ICP4 by granzyme B.
Liu, T., Khanna, K. M., Carriere, B. N. & Hendricks, R. L. γ interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J. Virol. 75, 11178–11184 (2001).
Liu, T., Khanna, K. M., Chen, X., Fink, D. J. & Hendricks, R. L. CD8+ T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J. Exp. Med. 191, 1459–1466 (2000).
Prabhakaran, K. et al. Sensory neurons regulate the effector functions of CD8+ T cells in controlling HSV-1 latency ex vivo. Immunity 23, 515–525 (2005).
Zhu, J. et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nature Med. 15, 886–892 (2009). This study shows that HSV-2 infection results in the formation of persistent localized CD4+ and CD8+ T cell foci in the dermis below the healed lesion, and that such foci can be readily infected by HIV-1.
Johansson, M. & Lycke, N. Immunological memory in B-cell-deficient mice conveys long-lasting protection against genital tract infection with Chlamydia trachomatis by rapid recruitment of T cells. Immunology 102, 199–208 (2001).
Johansson, E. L., Rudin, A., Wassen, L. & Holmgren, J. Distribution of lymphocytes and adhesion molecules in human cervix and vagina. Immunology 96, 272–277 (1999).
Zhu, J. et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J. Exp. Med. 204, 595–603 (2007). This study assessed CD8+ T cell surveillance of reactivating HSV-2 in humans, and showed that persistent CD8+ T cells and sensory nerve endings could quickly respond to and eliminate reactivated virus before it underwent extensive replication.
Schiller, J. T., Castellsague, X., Villa, L. L. & Hildesheim, A. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trial results. Vaccine 26 (Suppl. 10), K53–K61 (2008).
Campo, M. S. & Roden, R. B. Papillomavirus prophylactic vaccines: established successes, new approaches. J. Virol. 84, 1214–1220 (2010).
Mascola, J. R. & Montefiori, D. C. The role of antibodies in HIV vaccines. Annu. Rev. Immunol. 28, 413–444 (2010).
Stanberry, L. R. Clinical trials of prophylactic and therapeutic herpes simplex virus vaccines. Herpes 11 (Suppl. 3), 161A–169A (2004).
Virgin, H. W. & Walker, B. D. Immunology and the elusive AIDS vaccine. Nature 464, 224–231 (2010).
Gebhardt, T. et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nature Immunol. 10, 524–530 (2009). This study shows that memory CD8+ T cells that persist in the skin in primary HSV-1 infection provide enhanced clearance of virus on secondary challenge.
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Masopust, D., Vezys, V., Marzo, A. L. & Lefrancois, L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417 (2001).
Reinhardt, R. L., Khoruts, A., Merica, R., Zell, T. & Jenkins, M. K. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410, 101–105 (2001).
Klonowski, K. D. et al. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity 20, 551–562 (2004). This study analysed the migratory behaviour of CD8+ memory T cells, which showed controlled gating for entry into certain tissues, including the brain, peritoneum and intestinal lamina propria.
Reboldi, A. et al. C-C chemokine receptor 6-regulated entry of TH17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nature Immunol. 10, 514–523 (2009). This study shows that T cell entry into a restricted tissue occurs in two steps: a small number of pioneering CCR6+ T cells trigger the entry of a second wave of T cells that migrate into the central nervous system and cause encephalomyelitis.
Brady, M. T. Newborn circumcision: routine or not routine, that is the question. Arch. Pediatr. Adolesc. Med. 164, 94–96 (2010).
Round, J. L. & Mazmanian, S. K. The gut microbiota shapes intestinal immune responses during health and disease. Nature Rev. Immunol. 9, 313–323 (2009).
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004).
Tezuka, H. et al. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature 448, 929–933 (2007).
Atarashi, K. et al. ATP drives lamina propria TH17 cell differentiation. Nature 455, 808–812 (2008).
Gaboriau-Routhiau, V. et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31, 677–689 (2009).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Caramalho, I. et al. Regulatory T cells selectively express Toll-like receptors and are activated by lipopolysaccharide. J. Exp. Med. 197, 403–411 (2003).
O'Mahony, C. et al. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NFκB activation. PLoS Pathog. 4, e1000112 (2008).
Hall, J. A. et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity 29, 637–649 (2008).
Van Damme, L. et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 360, 971–977 (2002).
Karim, Q. A. et al. Effectiveness and safety of Tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 19 Jul 2010 (doi:10.1126/science.1193748).
VanCott, T. C. et al. HIV-1 neutralizing antibodies in the genital and respiratory tracts of mice intranasally immunized with oligomeric gp160. J. Immunol. 160, 2000–2012 (1998).
Di Tommaso, A. et al. Induction of antigen-specific antibodies in vaginal secretions by using a nontoxic mutant of heat-labile enterotoxin as a mucosal adjuvant. Infect. Immun. 64, 974–979 (1996).
Livingston, J. B., Lu, S., Robinson, H. & Anderson, D. J. Immunization of the female genital tract with a DNA-based vaccine. Infect. Immun. 66, 322–329 (1998).
Klavinskis, L. S., Barnfield, C., Gao, L. & Parker, S. Intranasal immunization with plasmid DNA-lipid complexes elicits mucosal immunity in the female genital and rectal tracts. J. Immunol. 162, 254–262 (1999).
Parr, E. L. & Parr, M. B. Immune responses and protection against vaginal infection after nasal or vaginal immunization with attenuated herpes simplex virus type-2. Immunology 98, 639–645 (1999).
Morrison, L. A., Da Costa, X. J. & Knipe, D. M. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology 243, 178–187 (1998).
Gallichan, W. S. et al. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J. Immunol. 166, 3451–3457 (2001).
Gallichan, W. S. & Rosenthal, K. L. Specific secretory immune responses in the female genital tract following intranasal immunization with a recombinant adenovirus expressing glycoprotein B of herpes simplex virus. Vaccine 13, 1589–1595 (1995).
Kool, M. et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J. Exp. Med. 205, 869–882 (2008).
Mora, J. R. et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314, 1157–1160 (2006).
Li, Z. et al. Novel vaccination protocol with two live mucosal vectors elicits strong cell-mediated immunity in the vagina and protects against vaginal virus challenge. J. Immunol. 180, 2504–2513 (2008).
Suvas, P. K., Dech, H. M., Sambira, F., Zeng, J. & Onami, T. M. Systemic and mucosal infection program protective memory CD8 T cells in the vaginal mucosa. J. Immunol. 179, 8122–8127 (2007).
Cuburu, N. et al. Sublingual immunization with nonreplicating antigens induces antibody-forming cells and cytotoxic T cells in the female genital tract mucosa and protects against genital papillomavirus infection. J. Immunol. 183, 7851–7859 (2009).
Lehner, T. et al. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nature Med. 2, 767–775 (1996).
Kozlowski, P. A. et al. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J. Immunol. 169, 566–574 (2002).
Johansson, E. L., Wassen, L., Holmgren, J., Jertborn, M. & Rudin, A. Nasal and vaginal vaccinations have differential effects on antibody responses in vaginal and cervical secretions in humans. Infect. Immun. 69, 7481–7486 (2001).
Rudin, A., Riise, G. C. & Holmgren, J. Antibody responses in the lower respiratory tract and male urogenital tract in humans after nasal and oral vaccination with cholera toxin B subunit. Infect. Immun. 67, 2884–2890 (1999).
Looker, K. J., Garnett, G. P. & Schmid, G. P. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull. World Health Organ. 86, 805–812 (2008).
Acknowledgements
A.I. is a recipient of the Burroughs Wellcome Fund (BWF) Investigators in the Pathogenesis of Infectious Disease award. This work was supported by a National Institutes of Health (NIH) grant to A.I. (R01AI054359, R01AI062428, R01AI064705, R21AI083242 and R01AI081884). The author would like to thank N. Iijima, H. Shin, E. Foxman, Y. Kumamoto and R. Medzhitov for review of this manuscript.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Glossary
- Microfold cell
-
(M cell). A specialized epithelial cell that delivers antigens from the gut lumen directly to intraepithelial lymphocytes and subepithelial lymphoid tissues by transepithelial vesicular transport.
- Transformation zone
-
The area that surrounds the border that is located between the endocervix and the ectocervix. This is where the columnar epithelial cells of the endocervix meet the stratified squamous epithelial cells of the ectocervix. It is the most common area for cervical cancer to develop.
- Founder virus
-
A transmitted virus, or a virus that gives rise to all virus quasispecies in an infected individual.
- Goblet cell
-
A mucus-producing cell that is located in the epithelial cell lining of the intestine and lungs.
- Paneth cell
-
A cell that is present at the base of the crypts in the intestinal epithelium, which produces antimicrobial proteins and peptides, including phospholipase A2 and defensins.
- γδ T cells
-
T cells expressing a T cell receptor that consists of a γ-chain and a δ-chain. These T cells are present in the skin, vagina and intestinal epithelium as intraepithelial lymphocytes. Although the exact function of γδ T cells is unknown, it has been suggested that mucosal γδ T cells are involved in innate immune responses.
- Langerhans cells
-
A population of dendritic cells that are resident in the epidermal layer of the skin and in type II epithelia.
- Pattern recognition receptors
-
(PRRs). Host receptors (such as Toll-like receptors (TLRs) or NOD-like receptors (NLRs)) that can detect pathogen-associated molecular patterns and initiate signalling cascades, leading to an innate immune response. These receptors can be membrane bound (such as TLRs) or soluble cytoplasmic receptors (such as retinoic acid-inducible gene I (RIG-I), melanoma differentiation-associated protein 5 (MDA5) and NLRs).
- Virus-like particles
-
(VLPs). Virion-like structures that are formed from the self assembly of viral envelope or capsid proteins in vitro. VLPs are not infectious because they do not contain a viral genome.
- TCID50
-
A typical virus infectivity assay that quantifies the amount of virus that is required to produce a cytopathic effect in 50% of inoculated tissue culture cells.
- Cross-presentation
-
The mechanism by which certain antigen-presenting cells take up, process and present extracellular antigens on MHC class I molecules to stimulate CD8+ T cells.
- Targeted lymph node immunization
-
A subcutaneous immunization technique that aims to administer a vaccine in the proximity of the internal and external iliac lymph nodes. This technique has been proposed as a means to harvest the naturally existing imprinting mechanism for instructing effector lymphocytes to migrate to the tissue (for example, the genital and rectal mucosae) that is being drained by a particular lymph node.
Rights and permissions
About this article
Cite this article
Iwasaki, A. Antiviral immune responses in the genital tract: clues for vaccines. Nat Rev Immunol 10, 699–711 (2010). https://doi.org/10.1038/nri2836
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri2836
This article is cited by
-
Tissue-resident memory T cells in the urogenital tract
Nature Reviews Nephrology (2022)
-
Tissue-resident immunity in the female and male reproductive tract
Seminars in Immunopathology (2022)
-
The human memory T cell compartment changes across tissues of the female reproductive tract
Mucosal Immunology (2021)
-
HIV Pathogenesis in the Human Female Reproductive Tract
Current HIV/AIDS Reports (2021)
-
A role for the CCR5–CCL5 interaction in the preferential migration of HSV-2-specific effector cells to the vaginal mucosa upon nasal immunization
Mucosal Immunology (2019)