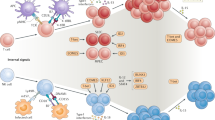
Abstract
Natural killer (NK) cells have roles in immunity and reproduction that are controlled by variable receptors that recognize MHC class I molecules. The variable NK cell receptors found in humans are specific to simian primates, in which they have progressively co-evolved with MHC class I molecules. The emergence of the MHC-C gene in hominids drove the evolution of a system of NK cell receptors for MHC-C molecules that is most elaborate in chimpanzees. By contrast, the human system of MHC-C receptors seems to have been subject to different selection pressures that have acted in competition on the immunological and reproductive functions of MHC class I molecules. We suggest that this compromise facilitated the development of the bigger brains that enabled archaic and modern humans to migrate out of Africa and populate other continents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Trowsdale, J. & Parham, P. Mini-review: defense strategies and immunity-related genes. Eur. J. Immunol. 34, 7–17 (2004).
Waterston, R. H. et al. Initial sequencing and comparative analysis of the mouse genome. Nature 420, 520–562 (2002).
Flajnik, M. F. & Kasahara, M. Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nature Rev. Genet. 11, 47–59 (2010).
Boehm, T. et al. VLR-based adaptive immunity. Annu. Rev. Immunol. 30, 203–220 (2012).
Moffett, A. & Loke, C. Immunology of placentation in eutherian mammals. Nature Rev. Immunol. 6, 584–594 (2006).
Vivier, E. et al. Innate or adaptive immunity? The example of natural killer cells. Science 331, 44–49 (2011).
Dos Reis, M. et al. Phylogenomic datasets provide both precision and accuracy in estimating the timescale of placental mammal phylogeny. Proc. Biol. Sci. 279, 3491–3500 (2012).
Lanier, L. L. NK cell recognition. Annu. Rev. Immunol. 23, 225–274 (2005).
Natarajan, K., Dimasi, N., Wang, J., Mariuzza, R. A. & Margulies, D. H. Structure and function of natural killer cell receptors: multiple molecular solutions to self, nonself discrimination. Annu. Rev. Immunol. 20, 853–885 (2002).
Parham, P. MHC class I molecules and KIRs in human history, health and survival. Nature Rev. Immunol. 5, 201–214 (2005).
Hoglund, P. & Brodin, P. Current perspectives of natural killer cell education by MHC class I molecules. Nature Rev. Immunol. 10, 724–734 (2010).
Sullivan, L. C., Clements, C. S., Rossjohn, J. & Brooks, A. G. The major histocompatibility complex class Ib molecule HLA-E at the interface between innate and adaptive immunity. Tissue Antigens 72, 415–424 (2008).
Weis, W. I., Taylor, M. E. & Drickamer, K. The C-type lectin superfamily in the immune system. Immunol. Rev. 163, 19–34 (1998).
Hammond, J. A., Guethlein, L. A., Abi-Rached, L., Moesta, A. K. & Parham, P. Evolution and survival of marine carnivores did not require a diversity of killer cell Ig-like receptors or Ly49 NK cell receptors. J. Immunol. 182, 3618–3627 (2009).
Guethlein, L. A., Abi-Rached, L., Hammond, J. A. & Parham, P. The expanded cattle KIR genes are orthologous to the conserved single-copy KIR3DX1 gene of primates. Immunogenetics 59, 517–522 (2007).
Dobromylskyj, M. & Ellis, S. Complexity in cattle KIR genes: transcription and genome analysis. Immunogenetics 59, 463–472 (2007).
Averdam, A. et al. A novel system of polymorphic and diverse NK cell receptors in primates. PLoS Genet. 5, e1000688 (2009).
Shum, B. P. et al. Conservation and variation in human and common chimpanzee CD94 and NKG2 genes. J. Immunol. 168, 240–252 (2002).
Bininda-Emonds, O. R. et al. The delayed rise of present-day mammals. Nature 446, 507–512 (2007).
Gumperz, J. E., Valiante, N. M., Parham, P., Lanier, L. L. & Tyan, D. Heterogeneous phenotypes of expression of the NKB1 natural killer cell class I receptor among individuals of different human histocompatibility leukocyte antigens types appear genetically regulated, but not linked to major histocompatibililty complex haplotype. J. Exp. Med. 183, 1817–1827 (1996).
Vilches, C. & Parham, P. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu. Rev. Immunol. 20, 217–251 (2002).
Cadavid, L. F. & Lun, C. M. Lineage-specific diversification of killer cell Ig-like receptors in the owl monkey, a New World primate. Immunogenetics 61, 27–41 (2009).
Perelman, P. et al. A molecular phylogeny of living primates. PLoS Genet. 7, e1001342 (2011).
Barrow, A. D. & Trowsdale, J. The extended human leukocyte receptor complex: diverse ways of modulating immune responses. Immunol. Rev. 224, 98–123 (2008).
Trowsdale, J. et al. The genomic context of natural killer receptor extended gene families. Immunol. Rev. 181, 20–38 (2001).
Wilson, M. J. et al. Plasticity in the organization and sequences of human KIR/ILT gene families. Proc. Natl Acad. Sci. USA 97, 4778–4783 (2000).
Guethlein, L. A., Older Aguilar, A. M., Abi-Rached, L. & Parham, P. Evolution of killer cell Ig-like receptor (KIR) genes: definition of an orangutan KIR haplotype reveals expansion of lineage III KIR associated with the emergence of MHC-C. J. Immunol. 179, 491–504 (2007).
Rajagopalan, S. Endosomal signaling and a novel pathway defined by the natural killer receptor KIR2DL4 (CD158d). Traffic 11, 1381–1390 (2010).
Moesta, A. K. et al. Synergistic polymorphism at two positions distal to the ligand-binding site makes KIR2DL2 a stronger receptor for HLA-C than KIR2DL3. J. Immunol. 180, 3969–3979 (2008).
Daza-Vamenta, R., Glusman, G., Rowen, L., Guthrie, B. & Geraghty, D. E. Genetic divergence of the rhesus macaque major histocompatibility complex. Genome Res. 14, 1501–1515 (2004).
Shiina, T. et al. Rapid evolution of major histocompatibility complex class I genes in primates generates new disease alleles in humans via hitchhiking diversity. Genetics 173, 1555–1570 (2006).
Adams, E. J. & Parham, P. Species-specific evolution of MHC class I genes in the higher primates. Immunol. Rev. 183, 41–64 (2001).
Bimber, B. N., Moreland, A. J., Wiseman, R. W., Hughes, A. L. & O'Connor, D. H. Complete characterization of killer Ig-like receptor (KIR) haplotypes in Mauritian cynomolgus macaques: novel insights into nonhuman primate KIR gene content and organization. J. Immunol. 181, 6301–6308 (2008).
Blokhuis, J. H., van der Wiel, M. K., Doxiadis, G. G. & Bontrop, R. E. The mosaic of KIR haplotypes in rhesus macaques. Immunogenetics 62, 295–306 (2010).
Kruse, P. H., Rosner, C. & Walter, L. Characterization of rhesus macaque KIR genotypes and haplotypes. Immunogenetics 62, 281–293 (2010).
Abi-Rached, L., Moesta, A. K., Rajalingam, R., Guethlein, L. A. & Parham, P. Human-specific evolution and adaptation led to major qualitative differences in the variable receptors of human and chimpanzee natural killer cells. PLoS Genet. 6, e1001192 (2010).
Older Aguilar, A. M. et al. Coevolution of killer cell Ig-like receptors with HLA-C to become the major variable regulators of human NK cells. J. Immunol. 185, 4238–4251 (2010).
Moesta, A. K., Abi-Rached, L., Norman, P. J. & Parham, P. Chimpanzees use more varied receptors and ligands than humans for inhibitory killer cell Ig-like receptor recognition of the MHC-C1 and MHC-C2 epitopes. J. Immunol. 182, 3628–3637 (2009).
Graef, T. et al. KIR2DS4 is a product of gene conversion with KIR3DL2 that introduced specificity for HLA-A*11 while diminishing avidity for HLA-C. J. Exp. Med. 206, 2557–2572 (2009).
Hilton, H. G. et al. Mutation at positively selected positions in the binding site for HLA-C shows that KIR2DL1 is a more refined but less adaptable NK cell receptor than KIR2DL3. J. Immunol. 189, 1418–1430 (2012).
Saulquin, X., Gastinel, L. N. & Vivier, E. Crystal structure of the human natural killer cell activating receptor KIR2DS2 (CD158j). J. Exp. Med. 197, 933–938 (2003).
Winter, C. C., Gumperz, J. E., Parham, P., Long, E. O. & Wagtmann, N. Direct binding and functional transfer of NK cell inhibitory receptors reveal novel patterns of HLA-C allotype recognition. J. Immunol. 161, 571–577 (1998).
Abi-Rached, L. et al. A small, variable, and irregular killer cell Ig-like receptor locus accompanies the absence of MHC-C and MHC-G in gibbons. J. Immunol. 184, 1379–1391 (2010).
Uhrberg, M. et al. Human diversity in killer cell inhibitory receptor genes. Immunity 7, 753–763 (1997).
Bari, R. et al. Significant functional heterogeneity among KIR2DL1 alleles and a pivotal role of arginine 245. Blood 114, 5182–5190 (2009).
Pyo, C. W. et al. Different patterns of evolution in the centromeric and telomeric regions of group A and B haplotypes of the human killer cell Ig-like receptor locus. PLoS ONE 5, e15115 (2010).
Anton, S. C. Natural history of Homo erectus. Am. J. Phys. Anthropol. 122 (Suppl. 37), 126–170 (2003).
Robson, S. L. & Wood, B. Hominin life history: reconstruction and evolution. J. Anat. 212, 394–425 (2008).
Hollenbach, J. A. et al. Report from the killer immunoglobulin-like receptor (KIR) anthropology component of the 15th International Histocompatibility Workshop: worldwide variation in the KIR loci and further evidence for the co-evolution of KIR and HLA. Tissue Antigens 76, 9–17 (2010).
Gendzekhadze, K. et al. Co-evolution of KIR2DL3 with HLA-C in a human population retaining minimal essential diversity of KIR and HLA class I ligands. Proc. Natl Acad. Sci. USA 106, 18692–18697 (2009).
King, A. et al. Evidence for the expression of HLAA-C class I mRNA and protein by human first trimester trophoblast. J. Immunol. 156, 2068–2076 (1996).
Apps, R. et al. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology 127, 26–39 (2009).
Moffett-King, A. Natural killer cells and pregnancy. Nature Rev. Immunol. 2, 656–663 (2002).
Bulmer, J. N. & Sunderland, C. A. Immunohistological characterization of lymphoid cell populations in the early human placental bed. Immunology 52, 349–357 (1984).
Hanna, J. et al. Decidual NK cells regulate key developmental processes at the human fetal–maternal interface. Nature Med. 12, 1065–1074 (2006).
King, A. & Loke, Y. W. Uterine large granular lymphocytes: a possible role in embryonic implantation? Am. J. Obstet. Gynecol. 162, 308–310 (1990).
Koopman, L. A. et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J. Exp. Med. 198, 1201–1212 (2003).
Manaster, I. & Mandelboim, O. The unique properties of uterine NK cells. Am. J. Reprod. Immunol. 63, 434–444 (2010).
Sharkey, A. M. et al. Killer Ig-like receptor expression in uterine NK cells is biased toward recognition of HLA-C and alters with gestational age. J. Immunol. 181, 39–46 (2008).
Trowsdale, J. & Moffett, A. NK receptor interactions with MHC class I molecules in pregnancy. Semin. Immunol. 20, 317–320 (2008).
Hiby, S. E. et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J. Clin. Invest. 120, 4102–4110 (2010).
Hiby, S. E. et al. Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage. Hum. Reprod. 23, 972–976 (2008).
Hiby, S. E. et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J. Exp. Med. 200, 957–965 (2004).
Schonberg, K., Sribar, M., Enczmann, J., Fischer, J. C. & Uhrberg, M. Analyses of HLA-C-specific KIR repertoires in donors with group A and B haplotypes suggest a ligand-instructed model of NK cell receptor acquisition. Blood 117, 98–107 (2011).
Chazara, O., Xiong, S. & Moffett, A. Maternal KIR and fetal HLA-C: a fine balance. J. Leukoc. Biol. 90, 703–716 (2011).
Single, R. M. et al. Global diversity and evidence for coevolution of KIR and HLA. Nature Genet. 39, 1114–1119 (2007).
Dring, M. M. et al. Innate immune genes synergize to predict increased risk of chronic disease in hepatitis C virus infection. Proc. Natl Acad. Sci. USA 108, 5736–5741 (2011).
Khakoo, S. I. et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science 305, 872–874 (2004).
Cheent, K. & Khakoo, S. I. Natural killer cells and hepatitis C: action and reaction. Gut 60, 268–278 (2010).
Wauquier, N., Padilla, C., Becquart, P., Leroy, E. & Vieillard, V. Association of KIR2DS1 and KIR2DS3 with fatal outcome in Ebola virus infection. Immunogenetics 62, 767–771 (2010).
McGrew, W. C. In search of the last common ancestor: new findings on wild chimpanzees. Phil. Trans. R. Soc. 365, 3267–3276 (2010).
White, T. D. et al. Ardipithecus ramidus and the paleobiology of early hominids. Science 326, 75–86 (2009).
DeGiorgio, M., Jakobsson, M. & Rosenberg, N. A. Out of Africa: modern human origins special feature: explaining worldwide patterns of human genetic variation using a coalescent-based serial founder model of migration outward from Africa. Proc. Natl Acad. Sci. USA 106, 16057–16062 (2009).
Crompton, R. H. et al. Human-like external function of the foot, and fully upright gait, confirmed in the 3.66 million year old Laetoli hominin footprints by topographic statistics, experimental footprint-formation and computer simulation. J. R. Soc. Interface 9, 707–719 (2012).
Franciscus, R. G. When did the modern human pattern of childbirth arise? New insights from an old Neandertal pelvis. Proc. Natl Acad. Sci. USA 106, 9125–9126 (2009).
Rosenberg, K. & Trevathan, W. Birth, obstetrics and human evolution. BJOG 109, 1199–1206 (2002).
Weaver, T. D. & Hublin, J. J. Neandertal birth canal shape and the evolution of human childbirth. Proc. Natl Acad. Sci. USA 106, 8151–8156 (2009).
Leonard, W. R., Snodgrass, J. J. & Robertson, M. L. Effects of brain evolution on human nutrition and metabolism. Annu. Rev. Nutr. 27, 311–327 (2007).
Vallender, E. J., Mekel-Bobrov, N. & Lahn, B. T. Genetic basis of human brain evolution. Trends Neurosci. 31, 637–644 (2008).
Carter, A. M. & Pijnenborg, R. Evolution of invasive placentation with special reference to non-human primates. Best Pract. Res. Clin. Obstet. Gynaecol. 25, 249–257 (2011).
DeSilva, J. & Lesnik, J. Chimpanzee neonatal brain size: implications for brain growth in Homo erectus. J. Hum. Evol. 51, 207–212 (2006).
Hublin, J. J. Out of Africa: modern human origins special feature: the origin of Neandertals. Proc. Natl Acad. Sci. USA 106, 16022–16027 (2009).
McDougall, I., Brown, F. H. & Fleagle, J. G. Stratigraphic placement and age of modern humans from Kibish, Ethiopia. Nature 433, 733–736 (2005).
Cann, R. L., Stoneking, M. & Wilson, A. C. Mitochondrial DNA and human evolution. Nature 325, 31–36 (1987).
Prugnolle, F., Manica, A. & Balloux, F. Geography predicts neutral genetic diversity of human populations. Curr. Biol. 15, R159–R160 (2005).
Moreau, C. et al. Deep human genealogies reveal a selective advantage to be on an expanding wave front. Science 334, 1148–1150 (2011).
Mulligan, C. J., Hunley, K., Cole, S. & Long, J. C. Population genetics, history, and health patterns in native americans. Annu. Rev. Genomics Hum. Genet. 5, 295–315 (2004).
Mourant, A. E., Kopec, A. C. & Domaniewska-Sobczak, K. The Distribution of the Human Blood Groups and Other Polymorphisms (Oxford Univ. Press, 1976).
Belich, M. P. et al. Unusual HLA-B alleles in two tribes of Brazilian Indians. Nature 357, 326–329 (1992).
Watkins, D. I. et al. New recombinant HLA-B alleles in a tribe of South American Amerindians indicate rapid evolution of MHC class I loci. Nature 357, 329–333 (1992).
Garber, T. L. et al. HLA-B alleles of the Cayapa of Ecuador: new B39 and B15 alleles. Immunogenetics 42, 19–27 (1995).
Little, A. M. et al. HLA class I diversity in Kolla Amerindians. Hum. Immunol. 62, 170–179 (2001).
Cadavid, L. F. & Watkins, D. I. Heirs of the jaguar and the anaconda: HLA, conquest and disease in the indigenous populations of the Americas. Tissue Antigens 50, 702–711 (1997).
Parham, P. et al. Episodic evolution and turnover of HLA-B in the indigenous human populations of the Americas. Tissue Antigens 50, 219–232 (1997).
Mellars, P. Neanderthals and the modern human colonization of Europe. Nature 432, 461–465 (2004).
Mellars, P. & French, J. C. Tenfold population increase in Western Europe at the Neandertal-to-modern human transition. Science 333, 623–627 (2011).
Green, R. E. et al. A draft sequence of the Neandertal genome. Science 328, 710–722 (2010).
Abi-Rached, L. et al. The shaping of modern human immune systems by multiregional admixture with archaic humans. Science 334, 89–94 (2011).
Reich, D. et al. Denisova admixture and the first modern human dispersals into Southeast Asia and Oceania. Am. J. Hum. Genet. 89, 516–528 (2011).
Boyington, J. C. & Sun, P. D. A structural perspective on MHC class I recognition by killer cell immunoglobulin-like receptors. Mol. Immunol. 38, 1007–1021 (2002).
Kaiser, B. K., Pizarro, J. C., Kerns, J. & Strong, R. K. Structural basis for NKG2A/CD94 recognition of HLA-E. Proc. Natl Acad. Sci. USA 105, 6696–6701 (2008).
Petrie, E. J. et al. CD94–NKG2A recognition of human leukocyte antigen (HLA)-E bound to an HLA class I leader sequence. J. Exp. Med. 205, 725–735 (2008).
Hansasuta, P. et al. Recognition of HLA-A3 and HLA-A11 by KIR3DL2 is peptide-specific. Eur. J. Immunol. 34, 1673–1679 (2004).
Acknowledgements
The authors thank L. Guethlein (Stanford) for her invaluable contributions to drafting the figures and preparing the manuscript. The authors also thank G. Burton (Cambridge) for advice and helpful discussions. Research from P.P.'s laboratory that is reviewed in this article was supported by grants from the US National Institutes of Health. A.M.'s laboratory is supported by the Wellcome Trust, the British Heart Foundation and the Centre for Trophoblast Research, University of Cambridge.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Adaptive introgression
-
The process by which a mating between two species, or two geographically or culturally separated populations, leads to the acquisition of functionally advantageous gene variants. Under natural selection these new variants rise to much higher frequency in their new 'home' species than genes that provide little or no advantage.
- Balancing selection
-
For certain genetic traits there are two or more alternative forms that provide complementary functions that are sufficiently valuable that they are maintained as a balanced polymorphism in the population. The most obvious example of a balanced polymorphism is that between X and Y chromosomes. Without both women (XX) and men (XY) a population cannot survive to the next generation. HLA class I, KIR and numerous other immune-system genes are maintained as balanced polymorphisms.
- Genetic drift
-
A process associated with small populations in which random events, as opposed to natural selection, lead to a polymorphic variant either rising to high frequency and being fixed or being driven to low frequency and eliminated from the population.
- KIR lineages I, II, III and V
-
The evolution of gene families of the immune system is always associated with the individual members acquiring functional and structural differences that define different phylogenetic lineages. In the KIR gene family the different lineages are associated with recognition of different MHC class I ligands.
- Population bottlenecks
-
Periods during which a population suffers a substantial reduction in size, and as a consequence loses potentially valuable genetic diversity. Epidemics of infectious disease and conflicts between warring populations can create population bottlenecks.
- Prosimian primates
-
Modern primate species, such as lemurs and lorises, that more closely resemble ancestral primates than do the simian primates. With the exception of the Madagascar lemurs, they have largely been replaced by the simian primates and the extant species are nocturnal.
- Simian primates
-
Simian primates comprise monkeys, apes and humans. They are characterized by good eyesight and flexible hands and feet. The only nocturnal species of simian primate are the owl monkeys (Aotus spp.) of South and Central America.
Rights and permissions
About this article
Cite this article
Parham, P., Moffett, A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol 13, 133–144 (2013). https://doi.org/10.1038/nri3370
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3370
This article is cited by
-
Determination for KIR genotype and allele copy number via real-time quantitative PCR method
Immunogenetics (2024)
-
Pregnancy, a test case for immunology
Synthese (2024)
-
Comprehensive single-cell transcriptomic and proteomic analysis reveals NK cell exhaustion and unique tumor cell evolutionary trajectory in non-keratinizing nasopharyngeal carcinoma
Journal of Translational Medicine (2023)
-
Killer cell immunoglobulin receptor diversity and its relevance in the human host’s response to HIV infection in African populations
Translational Medicine Communications (2023)
-
Investigation of KIR/HLA relationship and other clinical variables after T-cell-replete haploidentical bone marrow transplantation in patients with acute myeloid leukemia (AML)
BMC Immunology (2023)