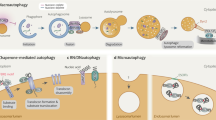
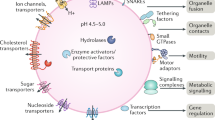
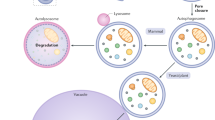
Key Points
-
Lysosomes are dynamic organelles that receive membrane traffic input from the secretory, endocytic, autophagic and phagocytic pathways. They can also fuse with the plasma membrane.
-
Live-cell imaging has shown that lysosomes interact with late endosomes by 'kiss-and-run' events and by direct fusion. Fusion results in the formation of hybrid organelles, in which the degradation of endocytosed macromolecules occurs and from which lysosomes are re-formed.
-
The use of yeast genetics and mammalian cell-free systems has identified much of the protein machinery that is involved in the delivery of macromolecules to lysosomes. The fusion of late endosomes with lysosomes involves tethering, the formation of trans-SNARE (soluble N-ethylmaleimide sensitive factor attachment protein receptor) complexes and phospholipid bilayer fusion.
-
Conventional lysosomes may fuse with the plasma membrane in response to a rise in cytosolic Ca2+ and can provide the additional membrane required for plasma-membrane wound repair. Specialized secretory lysosomes and lysosome-related organelles exist in some cell types.
-
Lysosomes may also fuse with phagosomes and autophagosomes. Some phagocytosed pathogens can prevent or delay phagolysosome biogenesis; others escape their intracellular vacuole by degrading the phagosomal membrane and may evade autophagy or reside in autophagic compartments and delay the formation of autolysosomes.
-
Upregulating autophagic pathways and the formation of autophagolysosomes provides the prospect of therapies for a range of proteinopathies including Huntington's disease and Parkinson's disease.
Abstract
Lysosomes are dynamic organelles that receive and degrade macromolecules from the secretory, endocytic, autophagic and phagocytic membrane-trafficking pathways. Live-cell imaging has shown that fusion with lysosomes occurs by both transient and full fusion events, and yeast genetics and mammalian cell-free systems have identified much of the protein machinery that coordinates these fusion events. Many pathogens that hijack the endocytic pathways to enter cells have evolved mechanisms to avoid being degraded by the lysosome. However, the function of lysosomes is not restricted to protein degradation: they also fuse with the plasma membrane during cell injury, as well as having more specialized secretory functions in some cell types.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
de Duve, C. The lysosome turns fifty. Nature Cell Biol. 7, 847–849 (2005). A perceptive, retrospective look at the discovery of lysosomes.
Holtzman, E. Lysosomes (Plenum, New York, 1989).
De Reuck, A. V. S. & Cameron, M. P. (eds). Ciba Foundation for the Promotion of International Cooperation in Medical and Chemical Research: Lysosomes (J. & A. Churchill, London, 1963).
Mellman, I., Fuchs, R. & Helenius, A. Acidification of the endocytic and exocytic pathways. Annu. Rev. Biochem. 55, 663–700 (1986).
Helenius, A., Mellman, I., Wall, D. & Hubbard, A. Endosomes. Trends Biochem. Sci. 8, 245–250 (1983).
Mellman, I. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12, 575–625 (1996).
Mukherjee, S., Ghosh, R. N. & Maxfield, F. R. Endocytosis. Physiol. Rev. 77, 759–803 (1997).
Storrie, B. & Desjardins, M. The biogenesis of lysosomes: is it a kiss and run, continuous fusion and fission process? Bioessays 18, 895–903 (1996).
Luzio, J. P. et al. Lysosome–endosome fusion and lysosome biogenesis. J. Cell Sci. 113, 1515–1524 (2000).
Mullins, C. & Bonifacino, J. S. The molecular machinery for lysosome biogenesis. Bioessays 23, 333–343 (2001).
Luzio, J. P. et al. Membrane dynamics and the biogenesis of lysosomes. Mol. Membr. Biol. 20, 141–154 (2003).
Bright, N. A., Gratian, M. J. & Luzio, J. P. Endocytic delivery to lysosomes mediated by concurrent fusion and kissing events in living cells. Curr. Biol. 15, 360–365 (2005). A study showing that the transfer of contents between endosomes and lysosomes occurs by kissing events and direct fusion in living cells.
Pryor, P. R., Mullock, B. M., Bright, N. A., Gray, S. R. & Luzio, J. P. The role of intraorganellar Ca2+ in late endosome–lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J. Cell Biol. 149, 1053–1062 (2000).
Ghosh, P., Dahms, N. M. & Kornfeld, S. Mannose 6-phosphate receptors: new twists in the tale. Nature Rev. Mol. Cell Biol. 4, 202–212 (2003).
Seaman, M. N. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J. Cell Biol. 165, 111–122 (2004).
Doray, B., Ghosh, P., Griffith, J., Geuze, H. J. & Kornfeld, S. Cooperation of GGAs and AP-1 in packaging MPRs at the trans-Golgi network. Science 297, 1700–1703 (2002).
Bonifacino, J. S. The GGA proteins: adaptors on the move. Nature Rev. Mol. Cell Biol. 5, 23–32 (2004).
Peden, A. A. et al. Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J. Cell Biol. 164, 1065–1076 (2004).
Ihrke, G., Kyttala, A., Russell, M. R., Rous, B. A. & Luzio, J. P. Differential use of two AP-3-mediated pathways by lysosomal membrane proteins. Traffic 5, 946–962 (2004).
Salazar, G. et al. BLOC-1 complex deficiency alters the targeting of adaptor protein complex-3 cargoes. Mol. Biol. Cell 17, 4014–4026 (2006).
Di Pietro, S. M. et al. BLOC-1 interacts with BLOC-2 and the AP-3 complex to facilitate protein trafficking on endosomes. Mol. Biol. Cell 17, 4027–4038 (2006).
Pak, Y., Glowacka, W. K., Bruce, M. C., Pham, N. & Rotin, D. Transport of LAPTM5 to lysosomes requires association with the ubiquitin ligase Nedd4, but not LAPTM5 ubiquitination. J. Cell Biol. 175, 631–645 (2006).
Haglund, K. et al. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nature Cell Biol. 5, 461–466 (2003).
Huang, F., Kirkpatrick, D., Jiang, X., Gygi, S. & Sorkin, A. Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol. Cell 21, 737–748 (2006).
Murphy, R. F. Maturation models for endosome and lysosome biogenesis. Trends Cell Biol. 1, 77–82 (1991).
Stoorvogel, W., Strous, G. J., Geuze, H. J., Oorschot, V. & Schwartz, A. L. Late endosomes derive from early endosomes by maturation. Cell 65, 417–427 (1991).
Gu, F. & Gruenberg, J. Biogenesis of transport intermediates in the endocytic pathway. FEBS Lett. 452, 61–66 (1999).
Rink, J., Ghigo, E., Kalaidzidis, Y. & Zerial, M. Rab conversion as a mechanism of progression from early to late endosomes. Cell 122, 735–749 (2005). A live-cell study showing the loss of RAB5 and recruitment of RAB7 during progression from early to late endosomes.
Gillooly, D. J. et al. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 19, 4577–4588 (2000).
White, I. J., Bailey, L. M., Aghakhani, M. R., Moss, S. E. & Futter, C. E. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J. 25, 1–12 (2006).
Gruenberg, J. & Stenmark, H. The biogenesis of multivesicular endosomes. Nature Rev. Mol. Cell Biol. 5, 317–323 (2004).
Bowers, K. & Stevens, T. H. Protein transport from the late Golgi to the vacuole in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1744, 438–454 (2005).
Slagsvold, T., Pattni, K., Malerod, L. & Stenmark, H. Endosomal and non-endosomal functions of ESCRT proteins. Trends Cell Biol. 16, 317–326 (2006).
Hurley, J. H. & Emr, S. D. The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu. Rev. Biophys. Biomol. Struct. 35, 277–298 (2006).
Kostelansky, M. S. et al. Molecular architecture and functional model of the complete yeast ESCRT-I heterotetramer. Cell 129, 485–498 (2007).
Chu, T., Sun, J., Saksena, S. & Emr, S. D. New component of ESCRT-I regulates endosomal sorting complex assembly. J. Cell Biol. 175, 815–823 (2006).
Oestreich, A. J., Davies, B. A., Payne, J. A. & Katzmann, D. J. Mvb12 is a novel member of ESCRT-I involved in cargo selection by the MVB pathway. Mol. Biol. Cell (2006).
Curtiss, M., Jones, C. & Babst, M. Efficient cargo sorting by ESCRT-I and the subsequent release of ESCRT-I from MVBs requires the subunit Mvb12. Mol. Biol. Cell (2006).
Horii, M. et al. CHMP7, a novel ESCRT-III-related protein, associates with CHMP4b and functions in the endosomal sorting pathway. Biochem. J. 400, 23–32 (2006).
Bowers, K. et al. Degradation of endocytosed epidermal growth factor and virally ubiquitinated major histocompatibility complex class I is independent of mammalian ESCRTII. J. Biol. Chem. 281, 5094–5105 (2006).
Langelier, C. et al. Human ESCRT-II complex and its role in human immunodeficiency virus type 1 release. J. Virol. 80, 9465–9480 (2006).
Bache, K. G. et al. The ESCRT-III subunit hVps24 is required for degradation but not silencing of the epidermal growth factor receptor. Mol. Biol. Cell 17, 2513–2523 (2006).
Hirst, J., Futter, C. E. & Hopkins, C. R. The kinetics of mannose 6-phosphate receptor trafficking in the endocytic pathway in HEp-2 cells: the receptor enters and rapidly leaves multivesicular endosomes without accumulating in a prelysosomal compartment. Mol. Biol. Cell 9, 809–816 (1998).
Aniento, F., Emans, N., Griffiths, G. & Gruenberg, J. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J. Cell Biol. 123, 1373–1387 (1993).
Ward, D. M., Pevsner, J., Scullion, M. A., Vaughn, M. & Kaplan, J. Syntaxin 7 and VAMP-7 are soluble N-ethylmaleimide-sensitive factor attachment protein receptors required for late endosome–lysosome and homotypic lysosome fusion in alveolar macrophages. Mol. Biol. Cell 11, 2327–2333 (2000).
Futter, C. E., Pearse, A., Hewlett, L. J. & Hopkins, C. R. Multivesicular endosomes containing internalized EGF–EGF receptor complexes mature and then fuse directly with lysosomes. J. Cell Biol. 132, 1011–1023 (1996). Electron-microscopic study that supports the concept of direct fusion between late endosomes and lysosomes and also demonstrates tethers between the organelles.
Bright, N. A., Reaves, B. J., Mullock, B. M. & Luzio, J. P. Dense core lysosomes can fuse with late endosomes and are re-formed from the resultant hybrid organelles. J. Cell Sci. 110, 2027–2040 (1997).
Mullock, B. M., Bright, N. A., Fearon, C. W., Gray, S. R. & Luzio, J. P. Fusion of lysosomes with late endosomes produces a hybrid organelle of intermediate density and is NSF dependent. J. Cell Biol. 140, 591–601 (1998).
van Deurs, B., Holm, P. K., Kayser, L. & Sandvig, K. Delivery to lysosomes in the human carcinoma cell line HEp-2 involves an actin filament-facilitated fusion between mature endosomes and preexisting lysosomes. Eur. J. Cell Biol. 66, 309–323 (1995).
Mullock, B. M., Branch, W. J., van Schaik, M., Gilbert, L. K. & Luzio, J. P. Reconstitution of an endosome–lysosome interaction in a cell-free system. J. Cell Biol. 108, 2093–2099 (1989).
Caplan, S., Hartnell, L. M., Aguilar, R. C., Naslavsky, N. & Bonifacino, J. S. Human Vam6p promotes lysosome clustering and fusion in vivo. J. Cell Biol. 154, 109–122 (2001).
Poupon, V., Stewart, A., Gray, S. R., Piper, R. C. & Luzio, J. P. The role of mVps18p in clustering, fusion, and intracellular localization of late endocytic organelles. Mol. Biol. Cell 14, 4015–4027 (2003).
Richardson, S. C., Winistorfer, S. C., Poupon, V., Luzio, J. P. & Piper, R. C. Mammalian late vacuole protein sorting orthologues participate in early endosomal fusion and interact with the cytoskeleton. Mol. Biol. Cell 15, 1197–1210 (2004).
Bucci, C., Thomsen, P., Nicoziani, P., McCarthy, J. & van Deurs, B. Rab7: a key to lysosome biogenesis. Mol. Biol. Cell 11, 467–480 (2000).
Marsman, M. et al. A splice variant of RILP induces lysosomal clustering independent of dynein recruitment. Biochem. Biophys. Res. Commun. 344, 747–756 (2006).
Jordens, I. et al. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein–dynactin motors. Curr. Biol. 11, 1680–1685 (2001).
Falcon-Perez, J. M., Nazarian, R., Sabatti, C. & Dell'Angelica, E. C. Distribution and dynamics of Lamp1-containing endocytic organelles in fibroblasts deficient in BLOC-3. J. Cell Sci. 118, 5243–5255 (2005).
Weber, T. et al. SNAREpins: minimal machinery for membrane fusion. Cell 92, 759–772 (1998).
Bock, J. B., Matern, H. T., Peden, A. A. & Scheller, R. H. A genomic perspective on membrane compartment organization. Nature 409, 839–841 (2001).
Yoshizawa, A. C. et al. Extracting sequence motifs and the phylogenetic features of SNARE-dependent membrane traffic. Traffic 7, 1104–1118 (2006).
Jahn, R. & Scheller, R. H. SNAREs — engines for membrane fusion. Nature Rev. Mol. Cell Biol. 7, 631–643 (2006).
Antonin, W. et al. A SNARE complex mediating fusion of late endosomes defines conserved properties of SNARE structure and function. EMBO J. 19, 6453–6464 (2000).
Antonin, W., Fasshauer, D., Becker, S., Jahn, R. & Schneider, T. R. Crystal structure of the endosomal SNARE complex reveals common structural principles of all SNAREs. Nature Struct. Biol. 9, 107–111 (2002).
Pryor, P. R. et al. Combinatorial SNARE complexes with VAMP7 or VAMP8 define different late endocytic fusion events. EMBO Rep. 5, 590–595 (2004). Identification of the SNAREs that are required for heterotypic fusion of late endosomes with lysosomes.
Martinez-Arca, S. et al. A dual mechanism controlling the localization and function of exocytic v-SNAREs. Proc. Natl Acad. Sci. USA 100, 9011–9016 (2003).
Griffiths, G. On vesicles and membrane compartments. Protoplasma 195, 37–58 (1996).
Mousavi, S. A., Brech, A., Berg, T. & Kjeken, R. Phosphoinositide 3-kinase regulates maturation of lysosomes in rat hepatocytes. Biochem. J. 372, 861–869 (2003).
Ko, D. C., Gordon, M. D., Jin, J. Y. & Scott, M. P. Dynamic movements of organelles containing Niemann–Pick C1 protein: NPC1 involvement in late endocytic events. Mol. Biol. Cell 12, 601–614 (2001).
Arighi, C. N., Hartnell, L. M., Aguilar, R. C., Haft, C. R. & Bonifacino, J. S. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J. Cell Biol. 165, 123–133 (2004).
Vergarajauregui, S. & Puertollano, R. Two di-leucine motifs regulate trafficking of mucolipin-1 to lysosomes. Traffic 7, 337–353 (2006).
Treusch, S. et al. Caenorhabditis elegans functional orthologue of human protein h-mucolipin-1 is required for lysosome biogenesis. Proc. Natl Acad. Sci. USA 101, 4483–4488 (2004).
Pryor, P. R., Reimann, F., Gribble, F. M. & Luzio, J. P. Mucolipin-1 is a lysosomal membrane protein required for intracellular lactosylceramide traffic. Traffic 7, 1388–1398 (2006).
Rodriguez, A., Webster, P., Ortego, J. & Andrews, N. W. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J. Cell Biol. 137, 93–104 (1997).
McNeil, P. L. & Kirchhausen, T. An emergency response team for membrane repair. Nature Rev. Mol. Cell Biol. 6, 499–505 (2005).
Andrade, L. O. & Andrews, N. W. The Trypanosoma cruzi–host-cell interplay: location, invasion, retention. Nature Rev. Microbiol. 3, 819–823 (2005).
Rao, S. K., Huynh, C., Proux-Gillardeaux, V., Galli, T. & Andrews, N. W. Identification of SNAREs involved in synaptotagmin VII-regulated lysosomal exocytosis. J. Biol. Chem. 279, 20471–20479 (2004). Identification of the SNAREs that are required for lysosome fusion with the plasma membrane.
Jaiswal, J. K., Chakrabarti, S., Andrews, N. W. & Simon, S. M. Synaptotagmin VII restricts fusion pore expansion during lysosomal exocytosis. PLoS Biol. 2, e233 (2004).
Chow, A., Toomre, D., Garrett, W. & Mellman, I. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature 418, 988–994 (2002).
Blott, E. J. & Griffiths, G. M. Secretory lysosomes. Nature Rev. Mol. Cell Biol. 3, 122–131 (2002).
Stinchcombe, J. C., Majorovits, E., Bossi, G., Fuller, S. & Griffiths, G. M. Centrosome polarization delivers secretory granules to the immunological synapse. Nature 443, 462–465 (2006). A study showing that movement of the centrosome in cytotoxic T lymphocytes is necessary for the delivery of secretory lysosomes to the immunological synapse for fusion with the plasma membrane.
Logan, M. R. et al. A critical role for vesicle-associated membrane protein-7 in exocytosis from human eosinophils and neutrophils. Allergy 61, 777–784 (2006).
Mollinedo, F. et al. Combinatorial SNARE complexes modulate the secretion of cytoplasmic granules in human neutrophils. J. Immunol. 177, 2831–2841 (2006).
Ren, Q. et al. Endobrevin/VAMP-8 is the primary v-SNARE for the platelet release reaction. Mol. Biol. Cell 18, 24–33 (2007).
Desjardins, M. Biogenesis of phagolysosomes: the 'kiss and run' hypothesis. Trends Cell Biol. 5, 183–186 (1995). A clear exposition of the hypothesis that phagosome maturation occurs as the result of series of 'kiss-and-run' events with endocytic organelles.
Touret, N. et al. Quantitative and dynamic assessment of the contribution of the ER to phagosome formation. Cell 123, 157–170 (2005).
Czibener, C. et al. Ca2+ and synaptotagmin VII-dependent delivery of lysosomal membrane to nascent phagosomes. J. Cell Biol. 174, 997–1007 (2006).
Jahraus, A. et al. In vitro fusion of phagosomes with different endocytic organelles from J774 macrophages. J. Biol. Chem. 273, 30379–30390 (1998). A demonstration of the fusogenic properties of phagosomes with endocytic organelles including lysosomes.
Mayorga, L. S., Bertini, F. & Stahl, P. D. Fusion of newly formed phagosomes with endosomes in intact cells and in a cell-free system. J. Biol. Chem. 266, 6511–6517 (1991).
Stockinger, W. et al. Differential requirements for actin polymerization, calmodulin, and Ca2+ define distinct stages of lysosome/phagosome targeting. Mol. Biol. Cell 17, 1697–1710 (2006).
Peyron, P., Maridonneau-Parini, I. & Stegmann, T. Fusion of human neutrophil phagosomes with lysosomes in vitro: involvement of tyrosine kinases of the Src family and inhibition by mycobacteria. J. Biol. Chem. 276, 35512–35517 (2001).
Harrison, R. E., Bucci, C., Vieira, O. V., Schroer, T. A. & Grinstein, S. Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: role of Rab7 and RILP. Mol. Cell. Biol. 23, 6494–6506 (2003). A demonstration of the role of RAB7 and microtubule motor proteins in promoting the extension of phagosomal tubules, which fuse with late endosomes or lysosomes.
Kjeken, R. et al. Fusion between phagosomes, early and late endosomes: a role for actin in fusion between late, but not early endocytic organelles. Mol. Biol. Cell 15, 345–358 (2004).
Huynh, K. K. et al. LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J. 26, 313–324 (2007).
Collins, R. F., Schreiber, A. D., Grinstein, S. & Trimble, W. S. Syntaxins 13 and 7 function at distinct steps during phagocytosis. J. Immunol. 169, 3250–3256 (2002).
Bogdanovic, A. et al. Syntaxin 7, syntaxin 8, Vti1 and VAMP7 (vesicle-associated membrane protein 7) form an active SNARE complex for early macropinocytic compartment fusion in Dictyostelium discoideum. Biochem. J. 368, 29–39 (2002).
Levine, B. & Klionsky, D. J. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev. Cell 6, 463–477 (2004).
Nakagawa, I. et al. Autophagy defends cells against invading group A Streptococcus. Science 306, 1037–1040 (2004). A clear demonstration that the autophagic machinery can act as an innate defence system against invading pathogens.
Darsow, T., Rieder, S. E. & Emr, S. D. A multispecificity syntaxin homologue, Vam3p, essential for autophagic and biosynthetic protein transport to the vacuole. J. Cell Biol. 138, 517–529 (1997).
Fischer von Mollard, G. & Stevens, T. H. The Saccharomyces cerevisiae v-SNARE Vti1p is required for multiple membrane transport pathways to the vacuole. Mol. Biol. Cell 10, 1719–1732 (1999).
Rieder, S. E. & Emr, S. D. A novel RING finger protein complex essential for a late step in protein transport to the yeast vacuole. Mol. Biol. Cell 8, 2307–2327 (1997).
Lindmo, K. et al. A dual function for Deep orange in programmed autophagy in the Drosophila melanogaster fat body. Exp. Cell Res. 312, 2018–2027 (2006).
Pulipparacharuvil, S. et al. Drosophila Vps16A is required for trafficking to lysosomes and biogenesis of pigment granules. J. Cell Sci. 118, 3663–3673 (2005).
Wang, C. W., Stromhaug, P. E., Shima, J. & Klionsky, D. J. The Ccz1–Mon1 protein complex is required for the late step of multiple vacuole delivery pathways. J. Biol. Chem. 277, 47917–47927 (2002).
Wang, C. W., Stromhaug, P. E., Kauffman, E. J., Weisman, L. S. & Klionsky, D. J. Yeast homotypic vacuole fusion requires the Ccz1–Mon1 complex during the tethering/docking stage. J. Cell Biol. 163, 973–985 (2003).
Jager, S. et al. Role for Rab7 in maturation of late autophagic vacuoles. J. Cell Sci. 117, 4837–4848 (2004).
Gonzalez-Polo, R. A. et al. The apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J. Cell Sci. 118, 3091–3102 (2005).
Behnia, R. & Munro, S. Organelle identity and the signposts for membrane traffic. Nature 438, 597–604 (2005).
Gruenberg, J. & van der Goot, F. G. Mechanisms of pathogen entry through the endosomal compartments. Nature Rev. Mol. Cell Biol. (2006).
Kim, K. J., Elliott, S. J., Di Cello, F., Stins, M. F. & Kim, K. S. The K1 capsule modulates trafficking of E. coli-containing vacuoles and enhances intracellular bacterial survival in human brain microvascular endothelial cells. Cell. Microbiol. 5, 245–252 (2003).
Brumell, J. H. & Grinstein, S. Salmonella redirects phagosomal maturation. Curr. Opin. Microbiol. 7, 78–84 (2004).
Deretic, V. et al. Mycobacterium tuberculosis inhibition of phagolysosome biogenesis and autophagy as a host defence mechanism. Cell. Microbiol. 8, 719–727 (2006).
Hernandez, L. D., Hueffer, K., Wenk, M. R. & Galan, J. E. Salmonella modulates vesicular traffic by altering phosphoinositide metabolism. Science 304, 1805–1807 (2004). Phagocytosed Salmonella secretes a phosphoinositide phosphatase that maintains high levels of phosphatidylinositol-3-phosphate in the phagosome membrane and delays formation of the phagolysosome.
Vergne, I., Chua, J. & Deretic, V. Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/calmodulin-PI3K hVPS34 cascade. J. Exp. Med. 198, 653–659 (2003).
Vergne, I. et al. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 102, 4033–4038 (2005).
Fratti, R. A., Backer, J. M., Gruenberg, J., Corvera, S. & Deretic, V. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J. Cell Biol. 154, 631–644 (2001).
Smith, A. C. et al. A network of Rab GTPases controls phagosome maturation and is modulated by Salmonella enterica serovar Typhimurium. J. Cell Biol. 176, 263–268 (2007).
Hamon, M., Bierne, H. & Cossart, P. Listeria monocytogenes: a multifaceted model. Nature Rev. Microbiol. 4, 423–434 (2006).
Cossart, P. et al. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect. Immun. 57, 3629–3636 (1989).
Shaughnessy, L. M., Hoppe, A. D., Christensen, K. A. & Swanson, J. A. Membrane perforations inhibit lysosome fusion by altering pH and calcium in Listeria monocytogenes vacuoles. Cell. Microbiol. 8, 781–792 (2006).
Henry, R. et al. Cytolysin-dependent delay of vacuole maturation in macrophages infected with Listeria monocytogenes. Cell. Microbiol. 8, 107–119 (2006).
Kirkegaard, K., Taylor, M. P. & Jackson, W. T. Cellular autophagy: surrender, avoidance and subversion by microorganisms. Nature Rev. Microbiol. 2, 301–314 (2004).
Gutierrez, M. G. et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119, 753–766 (2004).
Rich, K. A., Burkett, C. & Webster, P. Cytoplasmic bacteria can be targets for autophagy. Cell. Microbiol. 5, 455–468 (2003).
Ogawa, M. & Sasakawa, C. Intracellular survival of Shigella. Cell Microbiol 8, 177–184 (2006).
Ogawa, M. et al. Escape of intracellular Shigella from autophagy. Science 307, 727–731 (2005).
Ogawa, M. & Sasakawa, C. Bacterial evasion of the autophagic defense system. Curr. Opin. Microbiol. 9, 62–68 (2006).
Rubinsztein, D. C. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 443, 780–786 (2006). This paper reviews the role of autophagy in degrading aggregate-prone cytosolic proteins and proposes that upregulation of autophagy could be of therapeutic value in a range of neurodegenerative diseases.
Winchester, B. Lysosomal metabolism of glycoproteins. Glycobiology 15, 1R–15R (2005).
Artal-Sanz, M., Samara, C., Syntichaki, P. & Tavernarakis, N. Lysosomal biogenesis and function is critical for necrotic cell death in Caenorhabditis elegans. J. Cell Biol. 173, 231–239 (2006).
Arantes, R. M. & Andrews, N. W. A role for synaptotagmin VII-regulated exocytosis of lysosomes in neurite outgrowth from primary sympathetic neurons. J. Neurosci. 26, 4630–4637 (2006).
Lafont, F. et al. Raft association of SNAP receptors acting in apical trafficking in Madin–Darby canine kidney cells. Proc. Natl Acad. Sci. USA 96, 3734–3738 (1999).
Martinez-Arca, S., Alberts, P., Zahraoui, A., Louvard, D. & Galli, T. Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth. J. Cell Biol. 149, 889–900 (2000).
Merz, A. J. & Wickner, W. T. Trans-SNARE interactions elicit Ca2+ efflux from the yeast vacuole lumen. J. Cell Biol. 164, 195–206 (2004).
Acknowledgements
Unpublished experimental work referred to in this article was funded by a Research Grant to J.P.L from the Medical Research Council. The Cambridge Institute for Medical Research is supported by a Strategic Award from the Wellcome Trust. We thank M. Gratian for help with live-cell imaging and preparation of the lysosome–endosome animations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
OMIM
FURTHER INFORMATION
Glossary
- Endosome
-
A membrane-bound compartment (organelle) to which ligands, membrane components and fluid are delivered following internalization (endocytosis) from the cell surface.
- Membrane whorl
-
A membranous structure that has the appearance of being multi-lamellar or arranged in spirals when observed in cross-section.
- Autophagy
-
A process by which cytoplasmic components, including organelles, can be sequestered into autophagosomes and subsequently degraded.
- Phagosome
-
A membrane-bound compartment containing particles such as bacteria, yeast or parasites that have been internalized from the cell surface by the process of phagocytosis.
- Autophagosome
-
An intracellular compartment that is formed when a double membrane sequesters a portion of cytoplasm that often includes organelles. Autophagosomes subsequently fuse with lysosomes.
- trans-Golgi network
-
A tubulovesicular structure on the trans side of the stack of Golgi cisternae where cargo molecules are sorted to different secretory destinations.
- Multivesicular body
-
An endosome that contains many vesicles in the lumen of each organelle. It can also be called a late endosome.
- Dominant-negative mutant
-
A protein encoded by a mutated gene that prevents the function of the wild-type protein in cells in which both the mutant and wild-type proteins are expressed at the same time.
- Liposome
-
An artificial lipid vesicle that encloses an aqueous interior.
- Isopycnic ultracentrifugation
-
Centrifugation of samples (organelles or macromolecules) in a density gradient until an equilibrium is reached, such that the density of the sample is the same as that part of the density gradient in which it equilibrates.
- Retromer complex
-
A complex of cytoplasmic proteins that are required for some retrograde membrane-trafficking pathways that deliver cargo from endosomes to the trans-Golgi network.
- Parasitophorous vacuole
-
A membrane-bound organelle that contains an intracellular parasite; the membrane that surrounds the organelle is derived from the host cell but is modified by the parasite to facilitate its survival and growth.
- Melanosome
-
A membrane-bound organelle that contains melanin and is formed in melanocytes.
- Basophil granule
-
A granule in white blood cells that stains with basophilic dyes.
- Immunological synapse
-
A specialized contact area between a T lymphocyte and an antigen-presenting cell.
- Alpha granule
-
A platelet granule that contains several growth factors, clotting proteins and the adhesion molecule P-selectin.
- Phagosomal cup
-
A cup-shaped structure, formed principally by invagination of the plasma membrane during the early stages of phagocytic uptake of particles by cells. Membrane that originates from other organelles may be added to it.
- Macropinosome
-
A membrane-bound compartment (organelle), often of ∼0.5 μm diameter or larger. It is formed during fluid-phase uptake, particularly in regions of the cell where plasma-membrane ruffling occurs.
- α-2,8-linked polysialic acid
-
A linear homopolymer of N-acetyl neuraminic acid monomers that are linked by α-2,8 ketosidic linkages.
Rights and permissions
About this article
Cite this article
Luzio, J., Pryor, P. & Bright, N. Lysosomes: fusion and function. Nat Rev Mol Cell Biol 8, 622–632 (2007). https://doi.org/10.1038/nrm2217
Issue Date:
DOI: https://doi.org/10.1038/nrm2217
This article is cited by
-
Differential expression of miRNAs associated with pectoral myopathies in young broilers: insights from a comparative transcriptome analysis
BMC Genomics (2024)
-
The HEAT repeat protein HPO-27 is a lysosome fission factor
Nature (2024)
-
TMEM55B links autophagy flux, lysosomal repair, and TFE3 activation in response to oxidative stress
Nature Communications (2024)
-
Unexpected inhibition of the lipid kinase PIKfyve reveals an epistatic role for p38 MAPKs in endolysosomal fission and volume control
Cell Death & Disease (2024)
-
Development and validation of a novel lysosome-related LncRNA signature for predicting prognosis and the immune landscape features in colon cancer
Scientific Reports (2024)