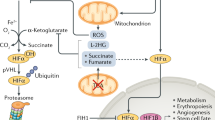
Key Points
-
Changes in oxygen (O2) tensions clearly have a role in patterning invertebrate and vertebrate embryos.
-
One of the first examples of the impact of O2 availability on developmental processes was demonstrated for the Drosophila melanogaster tracheal system. Here, O2-starved cells provide signals that promote increased branching morphogenesis of the O2-delivering tracheal network.
-
Genetic evidence now indicates that the differentiation of mammalian cardiovascular, placental, pulmonary, bone and haematopoietic cells and adipocytes is regulated by O2 availability.
-
Various pathways that mediate responses to O2 deprivation (hypoxia) have a role in embryonic development. The best genetic evidence is provided by animals that lack various subunits of the hypoxia inducible factor (HIF) heterodimeric transcription factors.
-
Recently, stem and progenitor cell phenotypes have been shown to be regulated by changes in O2 levels. Once again, HIFs have a crucial role in this process by interfacing with other stem cell signalling pathways such as those involving OCT4, Notch and Wnt.
Abstract
Low levels of oxygen (O2) occur naturally in developing embryos. Cells respond to their hypoxic microenvironment by stimulating several hypoxia-inducible factors (and other molecules that mediate O2 homeostasis), which then coordinate the development of the blood, vasculature, placenta, nervous system and other organs. Furthermore, embryonic stem and progenitor cells frequently occupy hypoxic 'niches' and low O2 regulates their differentiation. Recent work has revealed an important link between factors that are involved in regulating stem and progenitor cell behaviour and hypoxia-inducible factors, which provides a molecular framework for the hypoxic control of differentiation and cell fate. These findings have important implications for the development of therapies for tissue regeneration and disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Semenza, G. L. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu. Rev. Cell Dev. Biol. 15, 551–578 (1999).
Liu, L. & Simon, M. C. Regulation of transcription and translation by hypoxia. Cancer Biol. Ther. 3, 492–497 (2004).
Wouters, B. G. et al. Control of the hypoxic response through regulation of mRNA translation. Semin. Cell Dev. Biol. 16, 487–501 (2005).
Semenza, G. L. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol. Med. 7, 345–350 (2001).
Maltepe, E. & Simon, M. C. Oxygen, genes, and development: an analysis of the role of hypoxic gene regulation during murine vascular development. J. Mol. Med. 76, 391–401 (1998).
Simon, M. C. et al. Hypoxia, HIFs, and cardiovascular development. Cold Spring Harb. Symp. Quant. Biol. 67, 127–132 (2003).
Morriss, G. M. & New, D. A. Effect of oxygen concentration on morphogenesis of cranial neural folds and neural crest in cultured rat embryos. J. Embryol. Exp. Morphol. 54, 17–35 (1979).
Bruick, R. K. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 17, 2614–2623 (2003).
Guillemin, K. & Krasnow, M. A. The hypoxic response: huffing and HIFing. Cell 89, 9–12 (1997).
Manning, G. & Krasnow, M. A. Development of the Drosophila tracheal system. In The Development of Drosophila melanogaster (eds Bate, M. & Martinez-Arias, A.), 609–686 (Cold Spring Harbor Laboratory Press, New York,1993).
Samakovlis, C. et al. Development of the Drosophila tracheal system occurs by a series of morphologically distinct but genetically coupled branching events. Development 122, 1395–1407 (1996).
Jarecki, J., Johnson, E. & Krasnow, M. A. Oxygen regulation of airway branching in Drosophila is mediated by branchless FGF. Cell 99, 211–220 (1999). Elegantly describes a role for O 2 -starved cells in branching morphogenesis of the tracheal system so that deprived cells become fully oxygenated.
Shweiki, D., Itin, A., Soffer, D. & Keshet, E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359, 843–845 (1992). This is the first paper indicating that VEGF probably responds to O 2 deprivation to increase vascular density as a hypoxic adaptation.
Forsythe, J. A. et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 16, 4604–4613 (1996).
Ferrara, N., Gerber, H. P. & LeCouter, J. The biology of VEGF and its receptors. Nature Med. 9, 669–676 (2003).
Risau, W. Mechanisms of angiogenesis. Nature 386, 671–674 (1997).
Wood, S. M., Gleadle, J. M., Pugh, C. W., Hankinson, O. & Ratcliffe, P. J. The role of aryl hydrocarbon receptor nuclear translocator (ARNT) in hypoxia induction of gene expression. J. Biol. Chem. 271, 15117–15123 (1996).
Hirota, K. & Semenza, G. L. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit. Rev. Oncol. Hematol. 59, 15–26 (2006).
Manalo, D. J. et al. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105, 659–669 (2005).
Mitchell, J. A. & Yochim, J. M. Intrauterine oxygen tension during the estrous cycle in the rat: its relation to uterine respiration and vascular activity. Endocrinology 83, 701–705 (1968).
Rodesch, F., Simon, P., Donner, C. & Jauniaux, E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet. Gynecol. 80, 283–285 (1992).
Maltepe, E., Schmidt, J. V., Baunoch, D., Bradfield, C. A. & Simon, M. C. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature 386, 403–407 (1997). Shows that an environmental sensing bHLH–PAS protein regulates blood vessel morphogenesis in the developing conceptus.
Ramirez-Bergeron, D. L., Runge, A., Adelman, D. M., Gohil, M. & Simon, M. C. HIF-dependent hematopoietic factors regulate the development of the embryonic vasculature. Dev. Cell 11, 81–92 (2006).
Kozak, K. R., Abbott, B. & Hankinson, O. ARNT-deficient mice and placental differentiation. Dev. Biol. 191, 297–305 (1997).
Iyer, N. V. et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 12, 149–162 (1998).
Ryan, H. E., Lo, J. & Johnson, R. S. HIF-1α is required for solid tumor formation and embryonic vascularization. EMBO J. 17, 3005–3015 (1998). This article, along with reference 25, shows that the HIF pathway senses the low O 2 environment of the developing embryo to promote embryogenesis and angiogenesis.
Adelman, D. M., Maltepe, E. & Simon, M. C. Multilineage embryonic hematopoiesis requires hypoxic ARNT activity. Genes Dev. 13, 2478–2483 (1999).
Adelman, D. M., Gertsenstein, M., Nagy, A., Simon, M. C. & Maltepe, E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 14, 3191–3203 (2000).
Cowden Dahl, K. D. et al. Hypoxia-inducible factors 1α and 2α regulate trophoblast differentiation. Mol. Cell. Biol. 25, 10479–10491 (2005).
Gnarra, J. R. et al. Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc. Natl Acad. Sci. USA 94, 9102–9107 (1997).
Takeda, K. et al. Placental but not heart defects are associated with elevated hypoxia-inducible factor α levels in mice lacking prolyl hydroxylase domain protein 2. Mol. Cell. Biol. 26, 8336–8346 (2006).
Genbacev, O., Zhou, Y., Ludlow, J. W. & Fisher, S. J. Regulation of human placental development by oxygen tension. Science 277, 1669–1672 (1997). This article was the first to demonstrate that progenitor cell proliferation and differentiation is regulated in response to changes in O 2 availability.
Caniggia, I. et al. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFβ3 . J. Clin. Invest. 105, 577–587 (2000).
Tian, H., Hammer, R. E., Matsumoto, A. M., Russell, D. W. & McKnight, S. L. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 12, 3320–3324 (1998).
Compernolle, V. et al. Loss of HIF-2α and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nature Med. 8, 702–710 (2002).
Peng, J., Zhang, L., Drysdale, L. & Fong, G. H. The transcription factor EPAS-1/hypoxia-inducible factor 2α plays an important role in vascular remodeling. Proc. Natl. Acad. Sci. USA 97, 8386–8391 (2000).
Scortegagna, M. et al. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nature Genet. 35, 331–340 (2003).
Gruber, M. et al. Acute postnatal ablation of Hif-2α results in anemia. Proc. Natl Acad. Sci. USA 104, 2301–2306 (2007).
Rankin, E. B. et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J. Clin. Invest. 117, 1068–1077 (2007).
Isaac, D. D. & Andrew, D. J. Tubulogenesis in Drosophila: a requirement for the trachealess gene product. Genes Dev. 10, 103–117 (1996).
Wilk, R., Weizman, I. & Shilo, B.-Z. trachealess encodes a bHLH-PAS protein that is an inducer of tracheal cell fates in Drosophila. Genes Dev. 10, 93–102 (1996).
Rajpurohit, R., Koch, C. J., Tao, Z., Teixeira, C. M. & Shapiro, I. M. Adaptation of chondrocytes to low oxygen tension: relationship between hypoxia and cellular metabolism. J. Cell Physiol. 168, 424–432 (1996).
Erlebacher, A., Filvaroff, E. H., Gitelman, S. E. & Derynck, R. Toward a molecular understanding of skeletal development. Cell 80, 371–378 (1995).
Schipani, E. et al. Hypoxia in cartilage: HIF-1α is essential for chondrocyte growth arrest and survival. Genes Dev. 15, 2865–2876 (2001). This is among the first of many articles using a conditional allele of HIF1α to show that hypoxic microenvironments specifically regulate developing progenitor cells during organ formation.
Provot, S. et al. Hif-1α regulates differentiation of limb bud mesenchyme and joint development. J. Cell Biol. 177, 451–464 (2007).
Tontonoz, P., Hu, E., Devine, J., Beale, E. G. & Spiegelman, B. M. PPARγ2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene. Mol. Cell. Biol. 15, 351–357 (1995).
Yun, Z., Maecker, H. L., Johnson, R. S. & Giaccia, A. J. Inhibition of PPARγ2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev. Cell 2, 331–341 (2002).
Shimba, S., Wada, T., Hara, S. & Tezuka, M. EPAS1 promotes adipose differentiation in 3T3-L1 cells. J. Biol. Chem. 279, 40946–40953 (2004).
Mostafa, S. M., Papoutsakis, E. T. & Miller, W. M. Oxygen tension has significant effects on human megakaryocyte maturation. Exp. Hematology 28, 1498 (2000).
Morrison, S. J. et al. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J. Neurosci. 20, 7370–7376 (2000).
Studer, L. et al. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J. Neurosci. 20, 7377–7383 (2000).
Spradling, A., Drummond-Barbosa, D. & Kai, T. Stem cells find their niche. Nature 414, 98–104 (2001).
Lennon, D. P., Edmison, J. M. & Caplan, A. I. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: effects on in vitro and in vivo osteochondrogenesis. J. Cell Physiol. 187, 345–355 (2001).
Cipolleschi, M. G., Dello Sbarba, P. & Olivotto, M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood 82, 2031–2037 (1993).
Parmar, K., Mauch, P., Vergilio, J. A., Sackstein, R. & Down, J. D. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc. Natl Acad. Sci. USA 104, 5431–5436 (2007).
Danet, G. H., Pan, Y., Luongo, J. L., Bonnet, D. A. & Simon, M. C. Expansion of human SCID-repopulating cells under hypoxic conditions. J. Clin. Invest. 112, 126–135 (2003).
Kiel, M. J., Yilmaz, O. H., Iwashita, T., Terhorst, C. & Morrison, S. J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005). This was the first paper to suggest that stem and progenitor cells are associated with endothelial microenvironments.
Yoshida, S., Sukeno, M. & Nabeshima, Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science 317, 1722–1726 (2007).
Calabrese, C. et al. A perivascular niche for brain tumor stem cells. Cancer Cell 11, 69–82 (2007).
Harvey, A. J., Kind, K. L., Pantaleon, M., Armstrong, D. T. & Thompson, J. G. Oxygen-regulated gene expression in bovine blastocysts. Biol. Reprod. 71, 1108–1119 (2004).
Ezashi, T., Das, P. & Roberts, R. M. Low O2 tensions and the prevention of differentiation of hES cells. Proc. Natl Acad. Sci. USA 102, 4783–4788 (2005).
Jogi, A. et al. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc. Natl Acad. Sci. USA 99, 7021–7026 (2002).
Ramirez-Bergeron, D. L. et al. Hypoxia affects mesoderm and enhances hemangioblast specification during early development. Development 131, 4623–4634 (2004).
Artavanis-Tsakonas, S., Rand, M. D. & Lake, R. J. Notch signaling: cell fate control and signal integration in development. Science 284, 770–776 (1999).
Hansson, E. M., Lendahl, U. & Chapman, G. Notch signaling in development and disease. Semin. Cancer Biol. 14, 320–328 (2004).
Nofziger, D., Miyamoto, A., Lyons, K. M. & Weinmaster, G. Notch signaling imposes two distinct blocks in the differentiation of C2C12 myoblasts. Development 126, 1689–1702 (1999).
Dahlqvist, C. et al. Functional Notch signaling is required for BMP4-induced inhibition of myogenic differentiation. Development 130, 6089–6099 (2003).
Varnum-Finney, B. et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nature Med. 6, 1278–1281 (2000).
de la Pompa, J. L. et al. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development 124, 1139–1148 (1997).
Cornell, R. A. & Eisen, J. S. Delta/Notch signaling promotes formation of zebrafish neural crest by repressing neurogenin 1 function. Development 129, 2639–2648 (2002).
Kopan, R., Nye, J. S. & Weintraub, H. The intracellular domain of mouse Notch: a constitutively activated repressor of myogenesis directed at the basic helix-loop-helix region of MyoD. Development 120, 2385–2396 (1994).
Gustafsson, M. V. et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev. Cell 9, 617–628 (2005). Among the first to link the HIF and a stem cell pathway involving Notch.
Wang, R. et al. Transcriptional regulation of APH-1A and increased γ-secretase cleavage of APP and Notch by HIF-1 and hypoxia. FASEB J. 20, 1275–1277 (2006).
Kaidi, A., Williams, A. C. & Paraskeva, C. Interaction between β-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nature Cell Biol. 9, 210–217 (2007).
Covello, K. L. et al. HIF-2α regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 20, 557–570 (2006). This article first connected the pluripotent OCT4 transcription factor to changes in O 2 availability.
Nichols, J. et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95, 379–391 (1998).
Scholer, H. R., Ruppert, S., Suzuki, N., Chowdhury, K. & Gruss, P. New type of POU domain in germ line-specific protein Oct-4. Nature 344, 435–439 (1990).
Jiang, Y. et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418, 41–49 (2002).
Tai, M. H. et al. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis 26, 495–502 (2005).
Kehler, J. et al. Oct4 is required for primordial germ cell survival. EMBO Rep. 5, 1078–1083 (2004).
Lengner, C. J. et al. Oct4 expression is not required for mouse somatic stem cell self-renewal. Cell Stem Cell 1, 403–415 (2007).
Niwa, H., Miyazaki, J. & Smith, A. G. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nature Genet. 24, 372–376 (2000).
Hochedlinger, K., Yamada, Y., Beard, C. & Jaenisch, R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell 121, 465–477 (2005).
Hu, C.-J., Wang, L.-Y., Chodosh, L. A., Keith, B. & Simon, M. C. Differential roles of hypoxia-inducible factor 1α (HIF-1α) and HIF-2α in hypoxic gene regulation. Mol. Biol. Cell 23, 9361–9374 (2003).
Nordhoff, V. et al. Comparative analysis of human, bovine, and murine Oct-4 upstream promoter sequences. Mamm. Genome 12, 309–317 (2001).
Koshiji, M. et al. HIF-1α induces cell cycle arrest by functionally counteracting Myc. EMBO J. 23, 1949–1956 (2004).
Zhang, H. et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 11, 407–420 (2007).
Gordan, J. D., Bertout, J. A., Hu, C. J., Diehl, J. A. & Simon, M. C. HIF-2α promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell 11, 335–347 (2007).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006). The first in a series of papers showing that a relatively small number of factors can reprogramme fibroblasts into pluripotent cells.
Okita, K., Ichisaka, T. & Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 448, 313–317 (2007).
Maherali, N. et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 1, 55–70 (2007).
Wernig, M. et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448, 318–324 (2007).
Hochachka, P. W., Buck, L. T., Doll, C. J. & Land, S. C. Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc. Natl Acad. Sci. USA 93, 9493–9498 (1996). Among the first articles to show that O 2 deprivation is tolerated by altered intracellular metabolic pathways to conserve ATP.
Wullschleger, S., Loewith, R. & Hall, M. N. TOR signaling in growth and metabolism. Cell 124, 471–484 (2006).
Koumenis, C. & Wouters, B. G. “Translating” tumor hypoxia: unfolded protein response (UPR)-dependent and UPR-independent pathways. Mol. Cancer Res. 4, 423–436 (2006).
Gray, J. M. et al. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature 430, 317–322 (2004).
Guertin, D. A. & Sabatini, D. M. An expanding role for mTOR in cancer. Trends Mol. Med. 11, 353–361 (2005).
Sarbassov, D. D., Guertin, D. A., Ali, S. M. & Sabatini, D. M. Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science 307, 1098–1101 (2005).
Gangloff, Y. G. et al. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol. Cell. Biol. 24, 9508–9516 (2004).
Murakami, M. et al. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol. Cell. Biol. 24, 6710–6718 (2004).
Guertin, D. A. et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα, but not S6K1. Dev. Cell 11, 859–871 (2006).
Bi, L., Okabe, I., Bernard, D. J., Wynshaw-Boris, A. & Nussbaum, R. L. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110α subunit of phosphoinositide 3-kinase. J. Biol. Chem. 274, 10963–10968 (1999).
Lelievre, E., Bourbon, P. M., Duan, L. J., Nussbaum, R. L. & Fong, G. H. Deficiency in the p110α subunit of PI3K results in diminished Tie2 expression and Tie2−/−-like vascular defects in mice. Blood 105, 3935–3938 (2005).
Arsham, A. M., Howell, J. J. & Simon, M. C. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J. Biol. Chem. 278, 29655–29660 (2003).
Hudson, C. C. et al. Regulation of hypoxia-inducible factor 1α expression and function by the mammalian target of rapamycin. Mol. Cell. Biol. 22, 7004–7014 (2002).
Zhong, H. et al. Modulation of hypoxia-inducible factor 1α expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 60, 1541–1545 (2000).
Schroder, M. & Kaufman, R. J. The mammalian unfolded protein response. Annu. Rev. Biochem. 74, 739–789 (2005).
Koumenis, C. et al. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2α. Mol. Cell. Biol. 22, 7405–7416 (2002).
Harding, H. P. et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell. 7, 1153–1163 (2001).
Scheuner, D. et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell 7, 1165–1176 (2001).
Zhang, W. et al. PERK EIF2AK3 control of pancreatic β cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab. 4, 491–497 (2006).
Keith, B. & Simon, M. C. Hypoxia-inducible factors, stem cells, and cancer. Cell 129, 465–472 (2007).
Gu, Y. Z., Hogenesch, J. B. & Bradfield, C. A. The PAS superfamily: sensors of environmental and developmental signals. Annu. Rev. Pharmacol. Toxicol. 40, 519–561 (2000).
Wang, G. L. & Semenza, G. L. Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 270, 1230–1237 (1995).
Wang, G. L., Jiang, B.-H., Rue, E. A. & Semenza, G. L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl Acad. Sci. USA 92, 5510–5514 (1995).
Jain, S., Maltepe, E., Lu, M. M., Simon, C. & Bradfield, C. A. Expression of ARNT, ARNT2, HIF1α, HIF2α and Ah receptor mRNAs in the developing mouse. Mech. Dev. 73, 117–123 (1998).
Tian, H., McKnight, S. L. & Russell, D. W. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 11, 72–82 (1997).
Wiesener, M. S. et al. Widespread hypoxia-inducible expression of HIF-2α in distinct cell populations of different organs. FASEB J. 17, 271–273 (2003).
Gu, Y. Z., Moran, S. M., Hogenesch, J. B., Wartman, L. & Bradfield, C. A. Molecular characterization and chromosomal localization of a third α-class hypoxia inducible factor subunit, HIF3α. Gene Expr. 7, 205–213 (1998).
Makino, Y. et al. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature 414, 550–554 (2001).
Keith, B., Adelman, D. M. & Simon, M. C. Targeted mutation of the murine arylhydrocarbon receptor nuclear translocator 2 (Arnt2) gene reveals partial redundancy with Arnt. Proc. Natl Acad. Sci. USA 98, 6692–6697 (2001).
Bunger, M. K. et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103, 1009–1017 (2000).
Ivan, M. et al. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468 (2001).
Jaakkola, P. et al. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001).
Yu, F., White, S. B., Zhao, Q. & Lee, F. S. HIF-1α binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc. Natl Acad. Sci. USA 98, 9630–9635 (2001).
Masson, N., William, C., Maxwell, P. H., Pugh, C. W. & Ratcliffe, P. J. Independent function of two destruction domains in hypoxia-inducible factor-α chains activated by prolyl hydroxylation. EMBO J. 20, 5197–5206 (2001).
Epstein, A. C. et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 (2001).
Bruick, R. K. & McKnight, S. L. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294, 1337–1340 (2001).
Ivan, M. et al. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc. Natl Acad. Sci. USA 99, 13459–13464 (2002).
Schofield, C. J. & Ratcliffe, P. J. Oxygen sensing by HIF hydroxylases. Nature Rev. Mol. Cell Biol. 5, 343–354 (2004).
Ema, M. et al. Molecular mechanisms of transcription activation by HLF and HIF1α in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 18, 1905–1914 (1999).
Lando, D. et al. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 16, 1466–1471 (2002).
Lando, D., Peet, D. J., Whelan, D. A., Gorman, J. J. & Whitelaw, M. L. Asparagine hydroxylation of the HIF transactivation domain: a hypoxic switch. Science 295, 858–861 (2002).
Mahon, P. C., Hirota, K. & Semenza, G. L. FIH-1: a novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 15, 2675–2686 (2001).
Hewitson, K. S. et al. Hypoxia-inducible factor (HIF) asparagine hydroxylase is identical to factor inhibiting HIF (FIH) and is related to the cupin structural family. J. Biol. Chem. 277, 26351–26355 (2002).
Semenza, G. L. HIF-1 and human disease: one highly involved factor. Genes Dev. 14, 1983–1991 (2000).
Kaelin, W. G. Proline hydroxylation and gene expression. Annu. Rev. Biochem. 74, 115–128 (2005).
Bruick, R. K. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 17, 2614–2623 (2003).
Pear, W. S. & Simon, M. C. Lasting longer without oxygen: the influence of hypoxia on Notch signaling. Cancer Cell 8, 435–437 (2005).
Yoshida, H. ER stress and diseases. FEBS J. 274, 630–658 (2007).
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- Hypoxia
-
A state in which the level of O2 is decreased relative to the normal level (which is 2–9% in most mammalian cell types).
- Normoxic
-
Although frequently defined in the literature as 21% O2, physiological normoxia is actually in the range of 2–9% O2 for most adult cells in vivo.
- Ischaemia
-
A pathological condition resulting from blood vessel occlusion, involving deprivation of oxygen, nutrients and growth factors. This condition also usually leads to decreased tissue pH levels.
- Niche
-
The natural anatomic environment that supports stem cell behaviour.
- Ontogeny
-
The development of the fetus during embryogenesis.
- ETS
-
The founding member of a family of oncogenes and proto-oncogenes. ETS refers to 'E26-specific'.
- Ramification
-
The process of dividing or spreading into branches.
- Vasculogenesis
-
The formation of nascent blood vessels from newly generated endothelial cells.
- Angiogenesis
-
The remodelling of blood vessels into the large and small vessels that are typical of mature networks containing arteries, capillaries and veins.
- Somite
-
The primordial tissue that generates the vertebrae, dermis and muscles.
- Conceptus
-
An embryo or fetus.
- Cytotrophoblast
-
An outer cell of the developing embryo that adheres to the endometrium.
- Bradycardia
-
A slowing of heart rate, usually measured as fewer than 60 beats per minute in humans.
- Catecholamine dysregulation
-
Mice lacking HIF-2α die in utero owing to decreased production of catecholamine (for example, L-3,4-dihydroxyphenylalanine) by chromaffin cells in the organ of Zuckerkandl. Catecholamines are required for normal cardiovascular function.
- Arborize
-
To develop many branching parts or formations.
- Retinopathy
-
An abnormal increase in retinal vascular networks.
- Hepatic steatosis
-
The accumulation of lipid in the liver.
- Cardiac hypertrophy
-
Overgrowth of the heart through increased cell size rather than increased cell number.
- Skeletal myopathy
-
Any disease of the muscle tissues, such as muscular dystrophy.
- Physiological hypoxia
-
The natural low O2 level that is encountered by cells within the developing embryo, in particular before establishment of the utero–placental network.
- Adipogenesis
-
The differentiation of lipid-producing and storage cells known as adipocytes.
- Inner cell mass
-
Early cells in the embryo that generate all lineages of the mature organism but do not give rise to the placenta.
- Pluripotent
-
A cell that has the potential to differentiate into any of the cell lineages of the developing organism.
- Embryoid body
-
A three-dimensional structure consisting of differentiated derivatives of embryonic stem cells.
- Cancer stem cell
-
A cancer-initiating cell that can self-renew and generate distinct cell types.
Rights and permissions
About this article
Cite this article
Simon, M., Keith, B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol 9, 285–296 (2008). https://doi.org/10.1038/nrm2354
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm2354
This article is cited by
-
Mesenchymal stromal cells in hepatic fibrosis/cirrhosis: from pathogenesis to treatment
Cellular & Molecular Immunology (2023)
-
Cancer–nerve interplay in cancer progression and cancer-induced bone pain
Journal of Bone and Mineral Metabolism (2023)
-
Increased expression of 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase-3 is required for growth of mouse embryonic stem cells that are undergoing differentiation
Cytotechnology (2023)
-
Differential methylation in EGLN1 associates with blood oxygen saturation and plasma protein levels in high-altitude pulmonary edema
Clinical Epigenetics (2022)
-
Hypoxia is fine-tuned by Hif-1α and regulates mesendoderm differentiation through the Wnt/β-Catenin pathway
BMC Biology (2022)