Key Points
-
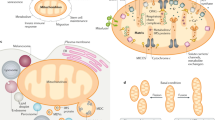
Cytochrome c is one of the mitochondrial proteins that is released into the cytosol when the cell is activated by an apoptotic stimulus.
-
In the cytosol, cytochrome c engages the apoptotic protease activating factor-1 (APAF1), and forms the apoptosome, which activates caspase-9.
-
The release of cytochrome c has been suggested to occur in two phases: mobilization from the mitochondrial intermembrane space and translocation through the outer mitochondrial membrane.
-
The mechanisms of cytochrome c release are controversial. Whether the permeabilization of the outer or the inner membrane is responsible for the downstream events is one of the debated topics. Most evidence supports a model in which the outer membrane is permeabilized without inner membrane events.
-
The release of cytochrome c and cytochrome-c-mediated apoptosis are controlled by multiple layers of regulation, with the most prominent players being members of the B-cell lymphoma protein-2 (BCL2) family.
Abstract
Cytochrome c is primarily known for its function in the mitochondria as a key participant in the life-supporting function of ATP synthesis. However, when a cell receives an apoptotic stimulus, cytochrome c is released into the cytosol and triggers programmed cell death through apoptosis. The release of cytochrome c and cytochrome-c-mediated apoptosis are controlled by multiple layers of regulation, the most prominent players being members of the B-cell lymphoma protein-2 (BCL2) family. As well as its role in canonical intrinsic apoptosis, cytochrome c amplifies signals that are generated by other apoptotic pathways and participates in certain non-apoptotic functions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Riedl, S. J. & Salvesen, G. S. The apoptosome: signalling platform of cell death. Nature Rev. Mol. Cell Biol. 8, 405–413 (2007).
Yuan, J. Divergence from a dedicated cellular suicide mechanism: exploring the evolution of cell death. Mol. Cell 23, 1–12 (2006).
Pellegrini, L. & Scorrano, L. A cut short to death: Parl and Opa1 in the regulation of mitochondrial morphology and apoptosis. Cell Death Differ. 14, 1275–1284 (2007).
Liu, X., Kim, C. N., Yang, J., Jemmerson, R. & Wang, X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86, 147–157 (1996). This study stunningly identified cytochrome c as a trigger for caspase activation, thereby hinting at the existence of the mitochondrial pathway of apoptosis.
Kluck, R. M. et al. Cytochrome c activation of CPP32-like proteolysis plays a critical role in a Xenopus cell-free apoptosis system. EMBO J. 16, 4639–4649 (1997).
Kluck, R. M., Bossy-Wetzel, E., Green, D. R. & Newmeyer, D. D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275, 1132–1136 (1997).
Yang, J. et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275, 1129–1132 (1997). This paper and reference 6 describe the ability of BCL2 to block OMM permeabilization as its mechanism of inhibiting apoptosis. These papers form the foundation of the studies of the mitochondrial pathway.
Newmeyer, D. D., Farschon, D. M. & Reed, J. C. Cell-free apoptosis in Xenopus egg extracts: inhibition by Bcl-2 and requirement for an organelle fraction enriched in mitochondria. Cell 79, 353–364 (1994). Provided the first evidence in a cell-free system that mitochondria have a biochemical role in apoptosis.
Li, F. et al. Cell-specific induction of apoptosis by microinjection of cytochrome c. Bcl-X L has activity independent of cytochrome c release. J. Biol. Chem. 272, 30299–30305 (1997).
Zhivotovsky, B., Orrenius, S., Brustugun, O. T. & Doskeland, S. O. Injected cytochrome c induces apoptosis. Nature 391, 449–450 (1998).
Li, K. et al. Cytochrome c deficiency causes embryonic lethality and attenuates stress-induced apoptosis. Cell 101, 389–399 (2000).
Vempati, U. D. et al. Role of cytochrome c in apoptosis: increased sensitivity to tumor necrosis factor α is associated with respiratory defects but not with lack of cytochrome c release. Mol. Cell. Biol. 27, 1771–1783 (2007).
Hao, Z. et al. Specific ablation of the apoptotic functions of cytochrome c reveals a differential requirement for cytochrome c and Apaf-1 in apoptosis. Cell 121, 579–591 (2005). Showed the in vivo significance of cytochrome c in apoptosis by using a knock-in approach in gene-targeted mice in which cytochrome c lacked its apoptotic activity but maintained its normal respiratory function.
Yu, T., Wang, X., Purring-Koch, C., Wei, Y. & McLendon, G. L. A mutational epitope for cytochrome c binding to the apoptosis protease activation factor-1. J. Biol. Chem. 276, 13034–13038 (2001).
Kluck, R. M. et al. Determinants of cytochrome c pro-apoptotic activity. The role of lysine 72 trimethylation. J. Biol. Chem. 275, 16127–16133 (2000).
Sharonov, G. V. et al. Comparative analysis of proapoptotic activity of cytochrome c mutants in living cells. Apoptosis 10, 797–808 (2005).
Abdullaev, Z. et al. A cytochrome c mutant with high electron transfer and antioxidant activities but devoid of apoptogenic effect. Biochem. J. 362, 749–754 (2002).
Ott, M., Zhivotovsky, B. & Orrenius, S. Role of cardiolipin in cytochrome c release from mitochondria. Cell Death Differ. 14, 1243–1247 (2007).
Gogvadze, V., Orrenius, S. & Zhivotovsky, B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim. Biophys. Acta 1757, 639–647 (2006).
Gonzalvez, F. & Gottlieb, E. Cardiolipin: setting the beat of apoptosis. Apoptosis 12, 877–885 (2007).
Dickerson, R. E. et al. Ferricytochrome c. I. General features of the horse and bonito proteins at 2.8 Å resolution. J. Biol. Chem. 246, 1511–1535 (1971).
Kalanxhi, E. & Wallace, C. J. Cytochrome c impaled: investigation of the extended lipid anchorage of a soluble protein to mitochondrial membrane models. Biochem. J. 407, 179–187 (2007).
Kagan, V. E. et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nature Chem. Biol. 1, 223–232 (2005).
Balakrishnan, G. et al. A conformational switch to β-sheet structure in cytochrome c leads to heme exposure. Implications for cardiolipin peroxidation and apoptosis. J. Am. Chem. Soc. 129, 504–505 (2007).
Garrido, C. et al. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 13, 1423–1433 (2006).
Kroemer, G., Galluzzi, L. & Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 87, 99–163 (2007).
Mootha, V. K. et al. A reversible component of mitochondrial respiratory dysfunction in apoptosis can be rescued by exogenous cytochrome c. EMBO J. 20, 661–671 (2001).
Zhao, Y., Wang, Z. B. & Xu, J. X. Effect of cytochrome c on the generation and elimination of O2•- and H2O2 in mitochondria. J. Biol. Chem. 278, 2356–2360 (2003).
Min, L. & Jian-xing, X. Detoxifying function of cytochrome c against oxygen toxicity. Mitochondrion 7, 13–16 (2007).
Orrenius, S., Gogvadze, V. & Zhivotovsky, B. Mitochondrial oxidative stress: implications for cell death. Annu. Rev. Pharmacol. Toxicol. 47, 143–183 (2007).
Uren, R. T. et al. Mitochondrial release of pro-apoptotic proteins: electrostatic interactions can hold cytochrome c but not Smac/DIABLO to mitochondrial membranes. J. Biol. Chem. 280, 2266–2274 (2005).
Munoz-Pinedo, C. et al. Different mitochondrial intermembrane space proteins are released during apoptosis in a manner that is coordinately initiated but can vary in duration. Proc. Natl Acad. Sci. USA 103, 11573–11578 (2006).
Goldstein, J. C., Waterhouse, N. J., Juin, P., Evan, G. I. & Green, D. R. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nature Cell Biol. 2, 156–162 (2000). This was the first paper to track OMM permeabilization during apoptosis in single cells, a feat that permitted an analysis of the sequence of events in this pathway.
Scorrano, L. et al. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev. Cell 2, 55–67 (2002). Describes a morphological change that occurs in mitochondria during apoptosis and suggests that this change is critical for the release of cytochrome c.
Sun, M. G. et al. Correlated three-dimensional light and electron microscopy reveals transformation of mitochondria during apoptosis. Nature Cell Biol. 9, 1057–1072 (2007).
Chipuk, J. E., Bouchier-Hayes, L. & Green, D. R. Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ. 13, 1396–1402 (2006).
Kuwana, T. et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111, 331–342 (2002). Showed that BAX permeabilizes lipid membranes sufficiently to allow large molecules to pass through, and that this effect requires an activation signal that is provided by BID. It also showed that specific lipids, such as cardiolipin, are implicated in the function of BAX.
Youle, R. J. & Strasser, A. The BCL-2 protein family: opposing activities that mediate cell death. Nature Rev. Mol. Cell Biol. 9, 47–59 (2008).
Wei, M. C. et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730 (2001). Describes the effects on mitochondria of BAX–BAK-double-knockout in mice and shows that BAX and BAK are the effectors of OMM permeabilization.
Chandra, D., Choy, G., Daniel, P. T. & Tang, D. G. Bax-dependent regulation of Bak by voltage-dependent anion channel 2. J. Biol. Chem. 280, 19051–19061 (2005).
Kinnally, K. W. & Antonsson, B. A tale of two mitochondrial channels, MAC and PTP, in apoptosis. Apoptosis 12, 857–868 (2007).
Mikhailov, V. et al. Association of Bax and Bak homo-oligomers in mitochondria. Bax requirement for Bak reorganization and cytochrome c release. J. Biol. Chem. 278, 5367–5376 (2003).
Basanez, G. et al. Bax-type apoptotic proteins porate pure lipid bilayers through a mechanism sensitive to intrinsic monolayer curvature. J. Biol. Chem. 277, 49360–49365 (2002). Provided a model of how BAX and BAK permeabilize membranes: in a manner that relies on protein–lipid interactions and makes use of a pore composed predominantly of lipid rather than protein. Although these features have not been proved, the model is nevertheless useful for thinking about the nature of the BAX–BAK pore.
Terrones, O. et al. Lipidic pore formation by the concerted action of proapoptotic BAX and tBID. J. Biol. Chem. 279, 30081–30091 (2004).
Shimizu, S., Narita, M. & Tsujimoto, Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399, 483–487 (1999).
Lawen, A. Another piece of the puzzle of apoptotic cytochrome c release. Mol. Microbiol. 66, 553–556 (2007).
Cheng, E. H., Sheiko, T. V., Fisher, J. K., Craigen, W. J. & Korsmeyer, S. J. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 301, 513–517 (2003).
He, L. & Lemasters, J. J. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett. 512, 1–7 (2002).
Alcala, S., Klee, M., Fernandez, J., Fleischer, A. & Pimentel-Muinos, F. X. A high-throughput screening for mammalian cell death effectors identifies the mitochondrial phosphate carrier as a regulator of cytochrome c release. Oncogene 27, 44–54 (2008).
Grimm, S. & Brdiczka, D. The permeability transition pore in cell death. Apoptosis 12, 841–855 (2007).
Siskind, L. J. Mitochondrial ceramide and the induction of apoptosis. J. Bioenerg. Biomembr. 37, 143–153 (2005).
Belizario, J. E., Alves, J., Occhiucci, J. M., Garay-Malpartida, M. & Sesso, A. A mechanistic view of mitochondrial death decision pores. Braz. J. Med. Biol. Res. 40, 1011–1024 (2007).
Morales, A., Colell, A., Mari, M., Garcia-Ruiz, C. & Fernandez-Checa, J. C. Glycosphingolipids and mitochondria: role in apoptosis and disease. Glycoconj. J. 20, 579–588 (2004).
Garcia-Ruiz, C., Colell, A., Paris, R. & Fernandez-Checa, J. C. Direct interaction of GD3 ganglioside with mitochondria generates reactive oxygen species followed by mitochondrial permeability transition, cytochrome c release, and caspase activation. FASEB J. 14, 847–858 (2000).
Kristal, B. S. & Brown, A. M. Apoptogenic ganglioside GD3 directly induces the mitochondrial permeability transition. J. Biol. Chem. 274, 23169–23175 (1999).
Crompton, M. Bax, Bid and the permeabilization of the mitochondrial outer membrane in apoptosis. Curr. Opin. Cell Biol. 12, 414–419 (2000).
Green, D. R. & Kroemer, G. The pathophysiology of mitochondrial cell death. Science 305, 626–629 (2004).
Waterhouse, N. J. et al. Cytochrome c maintains mitochondrial transmembrane potential and ATP generation after outer mitochondrial membrane permeabilization during the apoptotic process. J. Cell Biol. 153, 319–328 (2001).
Green, D. R. At the gates of death. Cancer Cell 9, 328–330 (2006).
Eskes, R., Desagher, S., Antonsson, B. & Martinou, J. C. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol. 20, 929–935 (2000).
Desagher, S. et al. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 144, 891–901 (1999).
Kluck, R. M. et al. The pro-apoptotic proteins, Bid and Bax, cause a limited permeabilization of the mitochondrial outer membrane that is enhanced by cytosol. J. Cell Biol. 147, 809–822 (1999). References 61 and 62 show that the activation of BAX by active BID leads to its insertion and oligomerization, forming a basis for the mechanism of action of BAX and, by extension, BAK.
Kim, T. H. et al. Bid–cardiolipin interaction at mitochondrial contact site contributes to mitochondrial cristae reorganization and cytochrome c release. Mol. Biol. Cell 15, 3061–3072 (2004).
Giordano, A. et al. tBid induces alterations of mitochondrial fatty acid oxidation flux by malonyl-CoA-independent inhibition of carnitine palmitoyltransferase-1. Cell Death Differ. 12, 603–613 (2005).
Tyurin, V. A. et al. Interactions of cardiolipin and lyso-cardiolipins with cytochrome c and tBid: conflict or assistance in apoptosis. Cell Death Differ. 14, 872–875 (2007).
Park, M. S., Kim, B. S. & Devarajan, P. Hypoxia/re-oxygenation injury induces apoptosis of LLC-PK1 cells by activation of caspase-2. Pediatr. Nephrol. 22, 202–208 (2007).
Robertson, J. D. et al. Processed caspase-2 can induce mitochondria-mediated apoptosis independently of its enzymatic activity. EMBO Rep. 5, 643–648 (2004).
Basanez, G. et al. Pro-apoptotic cleavage products of Bcl-X L form cytochrome c-conducting pores in pure lipid membranes. J. Biol. Chem. 276, 31083–31091 (2001).
Chen, Q., Gong, B. & Almasan, A. Distinct stages of cytochrome c release from mitochondria: evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress induced apoptosis. Cell Death Differ. 7, 227–233 (2000).
Lei, X. et al. Gossypol induces Bax/Bak-independent activation of apoptosis and cytochrome c release via a conformational change in Bcl-2. FASEB J. 20, 2147–2149 (2006).
Lakhani, S. A. et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 311, 847–851 (2006).
Paul, C. et al. Hsp27 as a negative regulator of cytochrome c release. Mol. Cell. Biol. 22, 816–834 (2002).
Puthalakath, H. et al. Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science 293, 1829–1832 (2001).
Bruey, J. M. et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nature Cell Biol. 2, 645–652 (2000).
Steel, R. et al. Hsp72 inhibits apoptosis upstream of the mitochondria and not through interactions with Apaf-1. J. Biol. Chem. 279, 51490–51499 (2004).
Karbowski, M. et al. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 159, 931–938 (2002).
Cassidy-Stone, A. et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev. Cell 14, 193–204 (2008).
Parone, P. A. et al. Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis. Mol. Cell. Biol. 26, 7397–7408 (2006).
Bouillet, P. et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286, 1735–1738 (1999). Described BIM-knockout mice and indicated that BIM is an important protein in the control of apoptosis in several settings.
Puthalakath, H. et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129, 1337–1349 (2007). Described the regulation of BIM stability (the importance of which is indicated in reference 79) and the role of Ca2+ in its effects. It is essential reading for an accurate understanding of ER stress and Ca2+ signalling.
Jemmerson, R. et al. A conformational change in cytochrome c of apoptotic and necrotic cells is detected by monoclonal antibody binding and mimicked by association of the native antigen with synthetic phospholipid vesicles. Biochemistry 38, 3599–3609 (1999).
Martin, A. G. & Fearnhead, H. O. Apocytochrome c blocks caspase-9 activation and Bax-induced apoptosis. J. Biol. Chem. 277, 50834–50841 (2002).
Borutaite, V. & Brown, G. C. Mitochondrial regulation of caspase activation by cytochrome oxidase and tetramethylphenylenediamine via cytosolic cytochrome c redox state. J. Biol. Chem. 282, 31124–31130 (2007).
Carreras, M. C. & Poderoso, J. J. Mitochondrial nitric oxide in the signaling of cell integrated responses. Am. J. Physiol. Cell Physiol. 292, C1569–C1580 (2007).
Schonhoff, C. M., Gaston, B. & Mannick, J. B. Nitrosylation of cytochrome c during apoptosis. J. Biol. Chem. 278, 18265–18270 (2003).
Vlasova, I. I. et al. Nitric oxide inhibits peroxidase activity of cytochrome c• cardiolipin complex and blocks cardiolipin oxidation. J. Biol. Chem. 281, 14554–14562 (2006).
Konishi, A. et al. Involvement of histone H1.2 in apoptosis induced by DNA double-strand breaks. Cell 114, 673–688 (2003).
Yan, N. & Shi, Y. Histone H1.2 as a trigger for apoptosis. Nature Struct. Biol. 10, 983–985 (2003).
Chipuk, J. E. & Green, D. R. Dissecting p53-dependent apoptosis. Cell Death Differ. 13, 994–1002 (2006).
Ruiz-Vela, A. & Korsmeyer, S. J. Proapoptotic histone H1.2 induces CASP-3 and -7 activation by forming a protein complex with CYT c, APAF-1 and CASP-9. FEBS Lett. 581, 3422–3428 (2007).
Khodjakov, A., Rieder, C., Mannella, C. A. & Kinnally, K. W. Laser micro-irradiation of mitochondria: is there an amplified mitochondrial death signal in neural cells? Mitochondrion 3, 217–227 (2004).
Colell, A. et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell 129, 983–997 (2007).
Deshmukh, M., Kuida, K. & Johnson, E. M. Jr. Caspase inhibition extends the commitment to neuronal death beyond cytochrome c release to the point of mitochondrial depolarization. J. Cell Biol. 150, 131–143 (2000).
Wright, K. M., Vaughn, A. E. & Deshmukh, M. Apoptosome dependent caspase-3 activation pathway is non-redundant and necessary for apoptosis in sympathetic neurons. Cell Death Differ. 14, 625–633 (2007).
Martinou, I. et al. The release of cytochrome c from mitochondria during apoptosis of NGF-deprived sympathetic neurons is a reversible event. J. Cell Biol. 144, 883–889 (1999).
Mendes, C. S. et al. Cytochrome c-d regulates developmental apoptosis in the Drosophila retina. EMBO Rep. 7, 933–939 (2006).
Honarpour, N. et al. Adult Apaf-1-deficient mice exhibit male infertility. Dev. Biol. 218, 248–258 (2000).
Kim, R., Emi, M. & Tanabe, K. Role of mitochondria as the gardens of cell death. Cancer Chemother. Pharmacol. 57, 545–553 (2006).
Hegde, R. et al. Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts inhibitor of apoptosis protein-caspase interaction. J. Biol. Chem. 277, 432–438 (2002).
Modjtahedi, N., Giordanetto, F., Madeo, F. & Kroemer, G. Apoptosis-inducing factor: vital and lethal. Trends Cell Biol. 16, 264–272 (2006).
Krantic, S., Mechawar, N., Reix, S. & Quirion, R. Apoptosis-inducing factor: a matter of neuron life and death. Prog. Neurobiol. 81, 179–196 (2007).
Arnoult, D. et al. Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax/Bak-mediated permeabilization. EMBO J. 22, 4385–4399 (2003).
Hill, M. M., Adrain, C., Duriez, P. J., Creagh, E. M. & Martin, S. J. Analysis of the composition, assembly kinetics and activity of native Apaf-1 apoptosomes. EMBO J. 23, 2134–2145 (2004).
Bernardi, P. et al. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 273, 2077–2099 (2006).
Jurgensmeier, J. M. et al. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl Acad. Sci. USA 95, 4997–5002 (1998).
Siu, W. P., Pun, P. B., Latchoumycandane, C. & Boelsterli, U. A. Bax-mediated mitochondrial outer membrane permeabilization (MOMP), distinct from the mitochondrial permeability transition, is a key mechanism in diclofenac-induced hepatocyte injury: multiple protective roles of cyclosporin A. Toxicol. Appl. Pharmacol. 227, 451–461 (2007).
Lee, M. & Park, J. Regulation of NFAT activation: a potential therapeutic target for immunosuppression. Mol. Cells 22, 1–7 (2006).
Serfling, E. et al. NFAT transcription factors in control of peripheral T cell tolerance. Eur. J. Immunol. 36, 2837–2843 (2006).
Woodside, K. J. et al. Apoptosis of allospecifically activated human helper T cells is blocked by calcineurin inhibition. Transpl. Immunol. 15, 229–234 (2006).
Canning, M. T., Nay, S. L., Pena, A. V. & Yarosh, D. B. Calcineurin inhibitors reduce nuclear localization of transcription factor NFAT in UV-irradiated keratinocytes and reduce DNA repair. J. Mol. Histol. 37, 285–291 (2006).
Bao, Q. & Shi, Y. Apoptosome: a platform for the activation of initiator caspases. Cell Death Differ. 14, 56–65 (2007).
Ho, L. H., Read, S. H., Dorstyn, L., Lambrusco, L. & Kumar, S. Caspase-2 is required for cell death induced by cytoskeletal disruption. Oncogene 14 Jan 2008 (doi:10.1038/sj.onc.1211005)
Author information
Authors and Affiliations
Related links
Glossary
- Caspase
-
A signalling Cys protease that cleaves after Asp residues. Caspases have an important role in apoptosis. An initiator caspase cleaves and activates itself to initiate the apoptotic programme. An executioner caspase executes the apoptotic programme through the cleavage of an array of vital proteins.
- Extrinsic pathway
-
An apoptotic pathway that is mediated by the binding of an extracellular ligand to a transmembrane receptor.
- Intrinsic pathway
-
An apoptotic pathway in which the crucial step is the permeabilization of the outer mitochondrial membrane.
- Death-inducing signalling complex
-
(DISC). The signalling platform of the extrinsic pathway.
- Cristae junction
-
An inner membrane projection into the matrix by tubular structures of uniform diameter.
- Redox intermediate
-
An electron carrier in a reaction in which electrons are transferred from donor molecules to acceptor molecules.
- Apoptosome
-
A signalling platform that activates the intrinsic pathway.
- SMAC
-
A pro-apoptotic protein that is released from the intermembrane space that neutralizes the inhibitory activity of inhibitors of apoptosis proteins.
- Reactive oxygen species
-
(ROS). Byproducts of oxidative metabolism that are highly reactive owing to unpaired electrons.
- Mitochondrial outer membrane permeabilization
-
(MOMP). The permeabilization of the outer mitochondrial membrane to proteins.
- Caspase recruitment domain
-
(CARD). A homotypic protein interaction motif that consists of six α-helices.
- WD40
-
A sequence of ∼40 amino acids that usually ends with Try-Asp (W-D). This is found in some regulatory proteins.
- AAA+ family
-
A family of ATPases in which the defining feature is the formation of an oligomer that has a circular structure. This occurs as a result of one ATPase domain nested next to the ATPase domain of its neighbour.
- Permeability transition pore
-
(PTP). A large high-conductance multimeric complex that spans the outer and inner mitochondrial membranes. Its opening leads to mitochondrial permeability transition, a sudden increase of the permeability of the membrane to solutes.
- Adenine nucleotide translocator
-
(ANT). A carrier that is found in the inner mitochondrial membrane that transports ADP into, and ATP out of, the mitochondrial matrix. It is thought to be a component of the permeability transition pore.
- Cyclophilin D
-
(CypD). An enzyme that is found in the mitochondrial matrix and that catalyses the cis/trans-isomerization of prolyl peptide bonds. It is thought to be a component of the permeability transition pore.
- Membrane potential
-
(Δψm). The proton-motive force that results from the generation of a proton gradient across the inner mitochondrial membrane. This enables the F0F1 ATPase to synthesize ATP from ADP and inorganic phosphate as the protons flow spontaneously across the membrane.
- BH3-only proteins
-
Pro-apoptotic BCL2-family members that have only the third of four BCL2 homology domains.
- Heat-shock protein
-
A protein that functions as a molecular chaperone that is upregulated during stress.
- Rhomboid proteases
-
A class of highly hydrophobic proteases. They contain a Ser-protease catalytic dyad which suggests that they can cleave the transmembrane domains of integral membrane proteins.
- Apocytochrome c
-
The cytochrome c protein that is produced by translation and co-translational modification in the cytosol.
- Holocytochrome c
-
The mature cytochrome c protein that contains the haem moiety.
- Inhibitors of apoptosis proteins
-
(IAPs). A family of proteins that associates with and inhibits caspases. These are defined by baculovirus-repeat domains and, in some cases, a RING zinc-finger domain.
- Autophagy
-
A process in which the parts of a cell that are sequestered within double-membraned vacuoles are digested by lysosomal hydrolases.
Rights and permissions
About this article
Cite this article
Ow, YL., Green, D., Hao, Z. et al. Cytochrome c: functions beyond respiration. Nat Rev Mol Cell Biol 9, 532–542 (2008). https://doi.org/10.1038/nrm2434
Issue Date:
DOI: https://doi.org/10.1038/nrm2434
This article is cited by
-
ALCAT1-mediated abnormal cardiolipin remodelling promotes mitochondrial injury in podocytes in diabetic kidney disease
Cell Communication and Signaling (2024)
-
PGAM5 exacerbates acute renal injury by initiating mitochondria-dependent apoptosis by facilitating mitochondrial cytochrome c release
Acta Pharmacologica Sinica (2024)
-
The potential role of hydrogen sulfide in cancer cell apoptosis
Cell Death Discovery (2024)
-
HSK3486 Inhibits Colorectal Cancer Growth by Promoting Oxidative Stress and ATPase Inhibitory Factor 1 Activation
Digestive Diseases and Sciences (2024)
-
Multifaceted role of redox pattern in the tumor immune microenvironment regarding autophagy and apoptosis
Molecular Cancer (2023)