Abstract
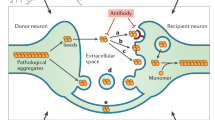
Neurodegenerative diseases are commonly associated with the accumulation of intracellular or extracellular protein aggregates. Recent studies suggest that these aggregates are capable of crossing cellular membranes and can directly contribute to the propagation of neurodegenerative disease pathogenesis. We propose that, once initiated, neuropathological changes might spread in a 'prion-like' manner and that disease progression is associated with the intercellular transfer of pathogenic proteins. The transfer of naked infectious particles between cells could therefore be a target for new disease-modifying therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Prusiner, S. B. Prions. Proc. Natl Acad. Sci. USA 95, 13363–13383 (1998).
Prusiner, S. B. Novel proteinaceous infectious particles cause scrapie. Science 216, 136–144 (1982).
Uversky, V. N. Neuropathology, biochemistry, and biophysics of α-synuclein aggregation. J. Neurochem. 103, 17–37 (2007).
Braak, H., Ghebremedhin, E., Rub, U., Bratzke, H. & Del Tredici, K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 318, 121–134 (2004).
Braak, H. et al. Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson's disease reconsidered. Mov. Disord. 21, 2042–2051 (2006).
Hawkes, C. H., Del Tredici, K. & Braak, H. Parkinson's disease: a dual-hit hypothesis. Neuropathol. Appl. Neurobiol. 33, 599–614 (2007).
Jang, H. et al. Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc. Natl Acad. Sci. USA 106, 14063–14068 (2009).
Jang, H., Boltz, D. A., Webster, R. G. & Smeyne, R. J. Viral parkinsonism. Biochim. Biophys. Acta 1792, 714–721 (2009).
Burke, R. E., Dauer, W. T. & Vonsattel, J. P. A critical evaluation of the Braak staging scheme for Parkinson's disease. Ann. Neurol. 64, 485–491 (2008).
Jellinger, K. A. Formation and development of Lewy pathology: a critical update. J. Neurol. 256 270–279 (2009).
Nelson, P. T., Braak, H. & Markesbery, W. R. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J. Neuropathol. Exp. Neurol. 68, 1–14 (2009).
Duyckaerts, C., Delatour, B. & Potier, M. C. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 118, 5–36 (2009).
Goedert, M., Klug, A. & Crowther, R. A. Tau protein, the paired helical filament and Alzheimer's disease. J. Alzheimers Dis. 9, 195–207 (2006).
Selkoe, D. J. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 81, 741–766 (2001).
Pearson, R. C., Esiri, M. M., Hiorns, R. W., Wilcock, G. K. & Powell, T. P. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc. Natl Acad. Sci. USA 82, 4531–4534 (1985).
Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991).
Delacourte, A. et al. Tau aggregation in the hippocampal formation: an ageing or a pathological process? Exp. Gerontol. 37, 1291–1296 (2002).
Lace, G. et al. Hippocampal tau pathology is related to neuroanatomical connections: an ageing population-based study. Brain 132, 1324–1334 (2009).
Arriagada, P. V., Growdon, J. H., Hedley-Whyte, E. T. & Hyman, B. T. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology 42, 631–639 (1992).
Cattaneo, E., Zuccato, C. & Tartari, M. Normal huntingtin function: an alternative approach to Huntington's disease. Nature Rev. Neurosci. 6, 919–930 (2005).
Davies, S. W. et al. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 90, 537–548 (1997).
DiFiglia, M. et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277, 1990–1993 (1997).
Scherzinger, E. et al. Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell 90, 549–558 (1997).
Duyao, M. et al. Trinucleotide repeat length instability and age of onset in Huntington's disease. Nature Genet. 4, 387–392 (1993).
Vonsattel, J. P. & DiFiglia, M. Huntington disease. J. Neuropathol. Exp. Neurol. 57, 369–384 (1998).
Deng, Y. P. et al. Differential loss of striatal projection systems in Huntington's disease: a quantitative immunohistochemical study. J. Chem. Neuroanat. 27, 143–164 (2004).
Kipps, C. M. et al. Progression of structural neuropathology in preclinical Huntington's disease: a tensor based morphometry study. J. Neurol. Neurosurg. Psychiatr. 76, 650–655 (2005).
Rosas, H. D. et al. Cerebral cortex and the clinical expression of Huntington's disease: complexity and heterogeneity. Brain 131, 1057–1068 (2008).
Brundin, P., Li, J. Y., Holton, J. L., Lindvall, O. & Revesz, T. Research in motion: the enigma of Parkinson's disease pathology spread. Nature Rev. Neursci. 9, 741–745 (2008).
Kordower, J. H., Chu, Y., Hauser, R. A., Freeman, T. B. & Olanow, C. W. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nature Med. 14, 504–506 (2008).
Kordower, J. H., Chu, Y., Hauser, R. A., Olanow, C. W. & Freeman, T. B. Transplanted dopaminergic neurons develop PD pathologic changes: a second case report. Mov. Disord. 23, 2303–2306 (2008).
Li, J. Y. et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nature Med. 14, 501–503 (2008).
Li, J. Y. et al. Characterization of Lewy body pathology in 12- and 16-year old intrastriatal mesencephalic grafts surviving in a patient with Parkinson's disease. Mov. Disord. 2 Mar 2010 (doi:10.1002/mds.23012).
Desplats, P. et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein. Proc. Natl Acad. Sci. USA 106, 13010–13015 (2009).
Lee, H. J. et al. Assembly-dependent endocytosis and clearance of extracellular α-synuclein. Int. J. Biochem. Cell Biol. 40, 1835–1849 (2008).
Danzer, K. M., Krebs, S. K., Wolff, M., Birk, G. & Hengerer, B. Seeding induced by α-synuclein oligomers provides evidence for spreading of α-synuclein pathology. J. Neurochem. 111, 192–203 (2009).
Danzer, K. M. et al. Different species of α-synuclein oligomers induce calcium influx and seeding. J. Neurosci. 27, 9220–9232 (2007).
Meyer-Luehmann, M. et al. Exogenous induction of cerebral β-amyloidogenesis is governed by agent and host. Science 313, 1781–1784 (2006).
Frost, B., Jacks, R. & Diamond, M. Propagation of tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 284, 12845–12852 (2009).
Clavaguera, F. et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nature Cell Biol. 11, 907–913 (2009).
Yang, W., Dunlap, J. R., Andrews, R. B. & Wetzel, R. Aggregated polyglutamine peptides delivered to nuclei are toxic to mammalian cells. Hum. Mol. Genet. 11, 2905–2917 (2002).
Ren, P. H. et al. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nature Cell Biol. 11, 219–225 (2009).
Rujano, M. A. et al. Polarised asymmetric inheritance of accumulated protein damage in higher eukaryotes. PLoS Biol. 4, e417 (2006).
Cicchetti, F. et al. Neural transplants in patients with Huntington's disease undergo disease-like neuronal degeneration. Proc. Natl Acad. Sci. USA 106, 12483–12488 (2009).
Vogiatzi, T., Xilouri, M., Vekrellis, K. & Stefanis, L. Wild type α-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J. Biol. Chem. 283, 23542–23556 (2008).
Jaiswal, J. K., Fix, M., Takano, T., Nedergaard, M. & Simon, S. M. Resolving vesicle fusion from lysis to monitor calcium-triggered lysosomal exocytosis in astrocytes. Proc. Natl Acad. Sci. USA 104, 14151–14156 (2007).
Lee, H. J., Patel, S. & Lee, S. J. Intravesicular localization and exocytosis of α-synuclein and its aggregates. J. Neurosci. 25, 6016–6024 (2005).
Morten, I. J., Gosal, W. S., Radford, S. E. & Hewitt, E. W. Investigation into the role of macrophages in the formation and degradation of β2-microglobulin amyloid fibrils. J. Biol. Chem. 282, 29691–29700 (2007).
Bucciantini, M. et al. Prefibrillar amyloid protein aggregates share common features of cytotoxicity. J. Biol. Chem. 279, 31374–31382 (2004).
van Rooijen, B. D., Claessens, M. M. & Subramaniam, V. Lipid bilayer disruption by oligomeric α-synuclein depends on bilayer charge and accessibility of the hydrophobic core. Biochim. Biophys. Acta 1788, 1271–1278 (2009).
Gousset, K. et al. Prions hijack tunnelling nanotubes for intercellular spread. Nature Cell Biol. 11, 328–336 (2009).
Brown, R. A brief account of microscopical observations made in the month of June, July and August, 1827, on the particles contained in the pollen of plants; and on the general existence of active molecules in organic and inorganic bodies. Phil. Mag. 4, 161–173 (1828).
Acknowledgements
All three investigators are supported by a joint Human Frontier Science Program grant on the topic relevant to this article. In addition, P.B. holds related grants from the MJ Fox Foundation for Parkinson's Research, Swedish Brain Foundation, Swedish Parkinson Foundation, Söderberg Foundation and the Swedish Research Council. R.R.K. is supported by the Huntington's disease Society of America Coalition for the Cure, the CHDI Foundation and the National Institute of Neurological Disease and Stroke. R.M. is supported by the Agence Nationale de la Recherche and the Centre National de la Recherche Scientifique. R.M. and P.B. are part of the ERA-net Neuron program MIPROTRAN.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
OMIM
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Brundin, P., Melki, R. & Kopito, R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol 11, 301–307 (2010). https://doi.org/10.1038/nrm2873
Issue Date:
DOI: https://doi.org/10.1038/nrm2873
This article is cited by
-
Modeling the neuroimmune system in Alzheimer’s and Parkinson’s diseases
Journal of Neuroinflammation (2024)
-
Scrutinizing the Therapeutic Potential of PROTACs in the Management of Alzheimer’s Disease
Neurochemical Research (2023)
-
Molecular Chaperones as Therapeutic Target: Hallmark of Neurodegenerative Disorders
Molecular Neurobiology (2023)
-
Connecting the Dots: Macromolecular Crowding and Protein Aggregation
Journal of Fluorescence (2023)
-
Transition of amyloid/mutant p53 from tumor suppressor to an oncogene and therapeutic approaches to ameliorate metastasis and cancer stemness
Cancer Cell International (2022)