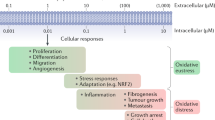
Key Points
-
Hydrogen sulfide (H2S) has emerged as an important gasotransmitter and signalling molecule, akin to nitric oxide (NO) and carbon monoxide (CO).
-
H2S is generated from Cys and its derivatives by three enzymes, cystathionine γ-lyase (CSE), cystathionine β-synthase (CBS) and 3-mercaptopyruvate sulfurtransferase (MST).
-
H2S has a major role in vasorelaxation and regulation of blood pressure. Mice lacking CSE display substantial cardiovascular dysfunction and hypertension.
-
One of the mechanisms by which H2S mediates its effects is via protein sulfhydration, which is analogous to nitrosylation by NO. In the process of sulfhydration, the thiol group of a reactive Cys is modified to an persulfide (-SSH) group, resulting in increased reactivity of the Cys residue. Sulfhydration is more prevalent than nitrosylation, as 25–50% of murine hepatic proteins were found to be sulfhydrated.
-
Sulfhydration orchestrates diverse signalling pathways ranging from vasorelaxation to the stress response. The vasoactivity of H2S stems from its ability to sulfhydrate the Kir6.1 subunit of ATP-dependent potassium (KATP) channels to elicit hyperpolarization of vascular smooth muscle cells.
-
H2S regulates the endoplasmic reticulum (ER) stress response by sulfhydrating and inhibiting protein Tyr phosphatase 1B (PTP1B), a key player in the ER stress response pathway. H2S also mediates the anti-apoptotic activity of nuclear factor-κB (NF-κB) by sulfhydrating the p65 subunit, thereby augmenting the transcription of pro-survival genes.
-
Sulfhydration is a reversible event, a feature that is necessary for the dynamic regulation of signalling pathways.
-
Sulhydration and nitrosylation may interface to modulate cellular responses. In the case of stress signalling by NF-κB, sulfhydration of Cys38 precedes its nitrosylation, providing a mechanistic basis for differentiating cell survival from cell death.
Abstract
Hydrogen sulfide (H2S) has recently emerged as a mammalian gaseous messenger molecule, akin to nitric oxide and carbon monoxide. H2S is predominantly formed from Cys or its derivatives by the enzymes cystathionine β-synthase and cystathionine γ-lyase. One of the mechanisms by which H2S signals is by sulfhydration of reactive Cys residues in target proteins. Although analogous to protein nitrosylation, sulfhydration is substantially more prevalent and usually increases the catalytic activity of targeted proteins. Physiological actions of sulfhydration include the regulation of inflammation and endoplasmic reticulum stress signalling as well as of vascular tension.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Stamler, J. S. et al. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc. Natl Acad. Sci. USA 89, 444–448 (1992). The first study demonstrating protein S -nitrosylation on Cys residues both in vitro and in vivo.
Szabo, C. Roles of hydrogen sulfide in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox Signal. 17, 68–80 (2012).
Szabo, C. & Papapetropoulos, A. Hydrogen sulphide and angiogenesis: mechanisms and applications. Br. J. Pharmacol. 164, 853–865 (2011).
Zhu, X. Y., Gu, H. & Ni, X. Hydrogen sulfide in the endocrine and reproductive systems. Expert Rev. Clin. Pharmacol. 4, 75–82 (2011).
Wang, R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev. 92, 791–896 (2012).
Bredt, D. S. & Snyder, S. H. Nitric oxide: a physiologic messenger molecule. Annu. Rev. Biochem. 63, 175–195 (1994).
Bredt, D. S. Nitric oxide signaling specificity — the heart of the problem. J. Cell Sci. 116, 9–15 (2003).
Verma, A., Hirsch, D. J., Glatt, C. E., Ronnett, G. V. & Snyder, S. H. Carbon monoxide: a putative neural messenger. Science 259, 381–384 (1993).
Zakhary, R. et al. Heme oxygenase 2: endothelial and neuronal localization and role in endothelium-dependent relaxation. Proc. Natl Acad. Sci. USA 93, 795–798 (1996).
Boehning, D. & Snyder, S. H. Novel neural modulators. Annu. Rev. Neurosci. 26, 105–131 (2003).
Boehning, D., Sedaghat, L., Sedlak, T. W. & Snyder, S. H. Heme oxygenase-2 is activated by calcium-calmodulin. J. Biol. Chem. 279, 30927–30930 (2004).
Stipanuk, M. H. & Beck, P. W. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem. J. 206, 267–277 (1982).
Shibuya, N., Mikami, Y., Kimura, Y., Nagahara, N. & Kimura, H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biochem. 146, 623–626 (2009).
Singh, S. & Banerjee, R. PLP-dependent H2S biogenesis. Biochim. Biophys. Acta 1814, 1518–1527 (2011).
Stipanuk, M. H. Metabolism of sulfur-containing amino acids. Annu. Rev. Nutr. 6, 179–209 (1986).
Chen, X., Jhee, K. H. & Kruger, W. D. Production of the neuromodulator H2S by cystathionine β-synthase via the condensation of cysteine and homocysteine. J. Biol. Chem. 279, 52082–52086 (2004).
Singh, S., Padovani, D., Leslie, R. A., Chiku, T. & Banerjee, R. Relative contributions of cystathionine β-synthase and γ-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J. Biol. Chem. 284, 22457–22466 (2009).
Beard, R. S. Jr & Bearden, S. E. Vascular complications of cystathionine β-synthase deficiency: future directions for homocysteine-to-hydrogen sulfide research. Am. J. Physiol. Heart Circ. Physiol. 300, H13–H26 (2011).
Sen, U., Mishra, P. K., Tyagi, N. & Tyagi, S. C. Homocysteine to hydrogen sulfide or hypertension. Cell Biochem. Biophys. 57, 49–58 (2010).
Watanabe, M. et al. Mice deficient in cystathionine β-synthase: animal models for mild and severe homocyst(e)inemia. Proc. Natl Acad. Sci. USA 92, 1585–1589 (1995).
Taoka, S., Ohja, S., Shan, X., Kruger, W. D. & Banerjee, R. Evidence for heme-mediated redox regulation of human cystathionine β-synthase activity. J. Biol. Chem. 273, 25179–25184 (1998).
Taoka, S., West, M. & Banerjee, R. Characterization of the heme and pyridoxal phosphate cofactors of human cystathionine β-synthase reveals nonequivalent active sites. Biochemistry 38, 2738–2744 (1999).
Banerjee, R. & Zou, C. G. Redox regulation and reaction mechanism of human cystathionine-β-synthase: a PLP-dependent hemesensor protein. Arch. Biochem. Biophys. 433, 144–156 (2005).
Shintani, T. et al. Cystathionine β-synthase as a carbon monoxide-sensitive regulator of bile excretion. Hepatology 49, 141–150 (2009).
Kabil, O. et al. Reversible heme-dependent regulation of human cystathionine β-synthase by a flavoprotein oxidoreductase. Biochemistry 50, 8261–8263 (2011).
Finkelstein, J. D., Kyle, W. E., Martin, J. L. & Pick, A. M. Activation of cystathionine synthase by adenosylmethionine and adenosylethionine. Biochem. Biophys. Res. Commun. 66, 81–87 (1975).
Abe, K. & Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 16, 1066–1071 (1996). The first evidence for a functional role of H 2 S in the brain.
Hishiki, T. et al. Carbon monoxide: impact on remethylation/transsulfuration metabolism and its pathophysiologic implications. J. Mol. Med. (Berl.) 90, 245–254 (2012).
Chiku, T. et al. H2S biogenesis by human cystathionine γ-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol. Chem. 284, 11601–11612 (2009).
Cavallini, D., Mondovi, B., De Marco, C. & Sciosciasantoro, A. Inhibitory effect of mercaptoethanol and hypotaurine on the desulfhydration of cysteine by cystathionase. Arch. Biochem. Biophys. 96, 456–457 (1962).
Szczepkowski, T. W. & Wood, J. L. The cystathionase-rhodanese system. Biochim. Biophys. Acta 139, 469–748 (1967).
Yang, G. et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine γ-lyase. Science 322, 587–590 (2008). The first study demonstrating a physiological role of H 2 S in vasorelaxation. This paper used CSE-deficient mice to establish that H 2 S is endogenously generated by CSE.
Diwakar, L. & Ravindranath, V. Inhibition of cystathionine-γ-lyase leads to loss of glutathione and aggravation of mitochondrial dysfunction mediated by excitatory amino acid in the CNS. Neurochem. Int. 50, 418–426 (2007).
Vitvitsky, V., Thomas, M., Ghorpade, A., Gendelman, H. E. & Banerjee, R. A functional transsulfuration pathway in the brain links to glutathione homeostasis. J. Biol. Chem. 281, 35785–35793 (2006).
Linden, D. R. et al. Production of the gaseous signal molecule hydrogen sulfide in mouse tissues. J. Neurochem. 106, 1577–1585 (2008).
Morikawa, T. et al. Hypoxic regulation of the cerebral microcirculation is mediated by a carbon monoxide-sensitive hydrogen sulfide pathway. Proc. Natl Acad. Sci. USA 109, 1293–1298 (2012). Demonstrates a crosstalk between the CO and H 2 S systems in regulation of cerebral vasodilation.
Enokido, Y. et al. Cystathionine β-synthase, a key enzyme for homocysteine metabolism, is preferentially expressed in the radial glia/astrocyte lineage of developing mouse CNS. FASEB J. 19, 1854–1856 (2005).
Lee, M., Schwab, C., Yu, S., McGeer, E. & McGeer, P. L. Astrocytes produce the antiinflammatory and neuroprotective agent hydrogen sulfide. Neurobiol. Aging 30, 1523–1534 (2009).
Dombkowski, R. A., Russell, M. J. & Olson, K. R. Hydrogen sulfide as an endogenous regulator of vascular smooth muscle tone in trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R678–R685 (2004).
Mathai, J. C. et al. No facilitator required for membrane transport of hydrogen sulfide. Proc. Natl Acad. Sci. USA 106, 16633–16638 (2009).
Czyzewski, B. K. & Wang, D. N. Identification and characterization of a bacterial hydrosulphide ion channel. Nature 483, 494–497 (2012).
Vitvitsky, V., Kabil, O. & Banerjee, R. High turnover rates for hydrogen sulfide allow for rapid regulation of its tissue concentrations. Antioxid. Redox Signal. 17, 22–31 (2012).
Toohey, J. I. Sulfur signaling: is the agent sulfide or sulfane? Anal. Biochem. 413, 1–7 (2011).
Pietri, R., Roman-Morales, E. & Lopez-Garriga, J. Hydrogen sulfide and hemeproteins: knowledge and mysteries. Antioxid. Redox Signal. 15, 393–404 (2011).
Weisiger, R. A., Pinkus, L. M. & Jakoby, W. B. Thiol S-methyltransferase: suggested role in detoxication of intestinal hydrogen sulfide. Biochem. Pharmacol. 29, 2885–7288 (1980).
Levitt, M. D., Furne, J., Springfield, J., Suarez, F. & DeMaster, E. Detoxification of hydrogen sulfide and methanethiol in the cecal mucosa. J. Clin. Invest. 104, 1107–1114 (1999).
Olson, K. R. A. Practical look at the chemistry and biology of hydrogen sulfide. Antioxid. Redox Signal. 17, 32–44 (2012).
Siegel, L. M. A. Direct microdetermination for sulfide. Anal. Biochem. 11, 126–132 (1965).
Levitt, M. D., Abdel-Rehim, M. S. & Furne, J. Free and acid-labile hydrogen sulfide concentrations in mouse tissues: anomalously high free hydrogen sulfide in aortic tissue. Antioxid. Redox Signal. 15, 373–378 (2011).
Kraus, D. W. & Doeller, J. E. Sulfide consumption by mussel gill mitochondria is not strictly tied to oxygen reduction: measurements using a novel polarographic sulfide sensor. J. Exp. Biol. 207, 3667–3679 (2004).
Kimura, H., Shibuya, N. & Kimura, Y. Hydrogen sulfide is a signaling molecule and a cytoprotectant. Antioxid. Redox Signal. 17, 45–57 (2012).
Jaffrey, S. R. & Snyder, S. H. The biotin switch method for the detection of S-nitrosylated proteins. Sci. STKE 2001, pl1 (2001).
Jaffrey, S. R., Erdjument-Bromage, H., Ferris, C. D., Tempst, P. & Snyder, S. H. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nature Cell Biol. 3, 193–197 (2001).
Mustafa, A. K. et al. H2S signals through protein S-sulfhydration. Sci. Signal. 2, ra72 (2009). The first study demonstrating protein sulfhydration in vivo by using a CSE-deficient mice model.
Sen, N. et al. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol. Cell 45, 13–24 (2012). Shows that pro-survival function of NF-κB is regulated by H 2 S, and introduces a new method using maleimide that can simultaneously measure sulfhydration and nitrosylation in the same sample.
Marino, S. M. & Gladyshev, V. N. Analysis and functional prediction of reactive cysteine residues. J. Biol. Chem. 287, 4419–4425 (2012).
Poole, L. B. & Nelson, K. J. Discovering mechanisms of signaling-mediated cysteine oxidation. Curr. Opin. Chem. Biol. 12, 18–24 (2008).
Klomsiri, C., Karplus, P. A. & Poole, L. B. Cysteine-based redox switches in enzymes. Antioxid. Redox Signal. 14, 1065–1077 (2011).
Finkel, T. From sulfenylation to sulfhydration: what a thiolate needs to tolerate. Sci. Signal. 5, pe10 (2012).
Krishnan, N., Fu, C., Pappin, D. J. & Tonks, N. K. H2S-induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci. Signal. 4, ra86 (2011). Links protein sulfhydration to ER stress signalling, and demonstrates that sulfhydration is reversible by the thioredoxin system.
Miller, D. L. & Roth, M. B. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 104, 20618–20622 (2007).
Benhar, M., Forrester, M. T. & Stamler, J. S. Protein denitrosylation: enzymatic mechanisms and cellular functions. Nature Rev. Mol. Cell Biol. 10, 721–732 (2009).
Hara, M. R. et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nature Cell Biol. 7, 665–674 (2005).
Sen, N. et al. Nitric oxide-induced nuclear GAPDH activates p300/CBP and mediates apoptosis. Nature Cell Biol. 10, 866–873 (2008).
Shatalin, K., Shatalina, E., Mironov, A. & Nudler, E. H2S: a universal defense against antibiotics in bacteria. Science 334, 986–990 (2011). Demonstrates that H 2 S acts as a bacterial cytoprotectant, implying that inhibitors of its biogenesis may facilitate antibiotic therapy.
Dickhout, J. G. et al. Integrated stress response modulates cellular redox state via induction of cystathionine γ-lyase: cross-talk between integrated stress response and thiol metabolism. J. Biol. Chem. 287, 7603–7614 (2012).
Harding, H. P. et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 (2003).
Szabo, C. Hydrogen sulphide and its therapeutic potential. Nature Rev. Drug Discov. 6, 917–935 (2007).
Yang, G. Hydrogen sulfide in cell survival: a double-edged sword. Expert Rev. Clin. Pharmacol. 4, 33–47 (2011).
Wallace, J. L., Caliendo, G., Santagada, V., Cirino, G. & Fiorucci, S. Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide-releasing diclofenac derivative in the rat. Gastroenterology 132, 261–271 (2007).
Wallace, J. L. Hydrogen sulfide-releasing anti-inflammatory drugs. Trends Pharmacol. Sci. 28, 501–505 (2007).
Moncada, S., Palmer, R. M. & Higgs, E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 43, 109–142 (1991).
Zakhary, R. et al. Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide. Proc. Natl Acad. Sci. USA 94, 14848–14853 (1997).
Pollock, J. S. et al. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc. Natl Acad. Sci. USA 88, 10480–10484 (1991).
Mustafa, A. K. et al. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ. Res. 109, 1259–1268 (2011).
Edwards, G., Feletou, M. & Weston, A. H. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch. 459, 863–879 (2010).
Wang, R. Hydrogen sulfide: a new EDRF. Kidney Int. 76, 700–704 (2009).
Prabhakar, N. R. & Semenza, G. L. Gaseous messengers in oxygen sensing. J. Mol. Med. (Berl.) 90, 265–272 (2012).
Krueger, D. et al. Signaling mechanisms involved in the intestinal pro-secretory actions of hydrogen sulfide. Neurogastroenterol. Motil. 22, 1224–1231, e319–e320 (2010).
Kohanski, M. A., Dwyer, D. J., Hayete, B., Lawrence, C. A. & Collins, J. J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130, 797–810 (2007).
Fu, M. et al. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc. Natl Acad. Sci. USA 109, 2943–2948 (2012).
Francis, S. H. Busch, J.L., Corbin, J.D. & Sibley, D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 62, 525–563 (2010).
Xue, L. et al. Carbon monoxide and nitric oxide as coneurotransmitters in the enteric nervous system: evidence from genomic deletion of biosynthetic enzymes. Proc. Natl Acad. Sci. USA 97, 1851–1855 (2000).
Bucci, M. et al. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler. Thromb. Vasc. Biol. 30, 1998–2004 (2010).
Acknowledgements
This work has been supported by United States Public Health Service Grants (MH018501) to S.H.S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Gasotransmitter
-
Endogenous gaseous molecule that can act as a messenger in signalling pathways.
- Homocysteinaemia
-
A disorder that is characterized by increased homocysteine levels in the blood.
- Astrocytes
-
Star-shaped glial cells of the central nervous system that form a structural and functional interface between non-nervous tissues and neurons.
- Sulfane sulfur
-
The uncharged form of sulfur (SO), which is attached to proteins through a covalent bond between the SO atom and other sulfur atoms.
- Amperometry
-
An electroanalytical technique that measures the current flow through an electrochemical cell. A constant fixed potential is applied to an electrochemical device (such as a sensor or electrode), and the current response, which reflects oxidation or reduction of analytes of interest, is monitored.
- Biotin switch assay
-
An assay that detects nitrosylation of Cys residues by replacing the NO moiety with a detectable biotin derivative.
- Acid dissociation constant
-
(pKa). A quantitative measure of the tendency of an acid to dissociate in solution. It is calculated as pKa = −log10Ka, in which Ka = [A−][H+]/[HA] and [A−], [H+] and [HA] are the concentration of the dissociated acid, protons and the undissociated (protonated) acid, respectively.
- Thioredoxin system
-
Maintains the reducing environment in cells by facilitating electron transfer from NADPH through thioredoxin reductase to thioredoxin, which reduces its target proteins using highly conserved thiol groups.
- Glyceraldehyde-3-phosphate dehydrogenase
-
(GAPDH). A glycolytic enzyme that catalyses the conversion of glyceraldehyde-3-phosphate to 1,3-bisphosphoglyceric acid.
- Endothelium-derived relaxing factors
-
(EDRFs). Factors derived from endothelial cells of blood vessels that can elicit vasorelaxation. Examples of EDRFs are NO, CO and H2S.
- Cholinergic stimuli
-
Refers to molecules that are capable of activating acetylcholine receptors.
- Muscarinic receptors
-
Acetylcholine G protein-coupled receptors that are activated by the prototypical agonist, muscarine, a compound isolated from the mushroom Amanita muscaria.
- Calmodulin
-
A calcium protein that is found in all eukaryotes. Calmodulin has a high degree of structural conservation, can bind to target enzymes and modulate their activity as a function of cytosolic calcium concentration.
- ATP-dependent potassium channels
-
(KATP channels). Potassium channels that are regulated by ATP. Binding of ATP keeps the channel in a closed conformation, preventing the entry of K+ ions.
- Endothelium-derived hyperpolarizing factors
-
(EDHFs). Substances derived from the endothelium that act via ion channels to increase the electronegativity of the membrane.
- Resistance blood vessels
-
Blood vessels that exhibit increased resistance to blood flow. Vascular resistance is directly proportional to the extent of vasoconstriction, which is one of the primary determinants of blood pressure.
- Normoxic conditions
-
Ambient O2 concentrations (∼21%) or, in reference to tissue, O2 concentrations in normal physiological condition (∼2–3%).
Rights and permissions
About this article
Cite this article
Paul, B., Snyder, S. H2S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol 13, 499–507 (2012). https://doi.org/10.1038/nrm3391
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3391
This article is cited by
-
Dissolving sodium hydrosulfide in drinking water is not a good source of hydrogen sulfide for animal studies
Scientific Reports (2023)
-
Discovery of a cystathionine γ-lyase (CSE) selective inhibitor targeting active-site pyridoxal 5′-phosphate (PLP) via Schiff base formation
Scientific Reports (2023)
-
Therapeutic Hypothermia Combined with Hydrogen Sulfide Treatment Attenuated Early Blood–Brain Barrier Disruption and Brain Edema Induced by Cardiac Arrest and Resuscitation in Rat Model
Neurochemical Research (2023)
-
The anti-inflammatory effect of dimethyl trisulfide in experimental acute pancreatitis
Scientific Reports (2023)
-
One-year follow-up study after patients with severe COVID-19 received human umbilical cord mesenchymal stem cells treatment
Stem Cell Research & Therapy (2022)