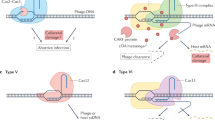
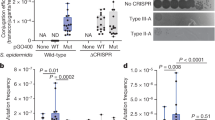
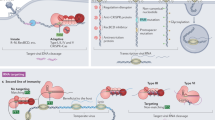
Abstract
The discovery of CRISPR–Cas (clustered, regularly interspaced short palindromic repeats–CRISPR-associated proteins) adaptive immune systems in prokaryotes has been one of the most exciting advances in microbiology in the past decade. Their role in host protection against mobile genetic elements is now well established, but there is mounting evidence that these systems modulate other processes, such as the genetic regulation of group behaviour and virulence, DNA repair and genome evolution. In this Progress article, we discuss recent studies that have provided insights into these unconventional CRISPR–Cas functions and consider their potential evolutionary implications. Understanding the role of CRISPR–Cas in these processes will improve our understanding of the evolution and maintenance of CRISPR–Cas systems in prokaryotic genomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Westra, E. R. et al. The CRISPRs, they are a-changin': how prokaryotes generate adaptive immunity. Annu. Rev. Genet. 46, 311–339 (2012).
Labrie, S. J., Samson, J. E. & Moineau, S. Bacteriophage resistance mechanisms. Nature Rev. Microbiol. 8, 317–327 (2010).
Wiedenheft, B., Sternberg, S. H. & Doudna, J. A. RNA-guided genetic silencing systems in bacteria and archaea. Nature 482, 331–338 (2012).
Makarova, K. S. et al. Evolution and classification of the CRISPR–Cas systems. Nature Rev. Microbiol. 9, 467–477 (2011).
Barrangou, R. et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–1712 (2007).
Andersson, A. F. & Banfield, J. F. Virus population dynamics and acquired virus resistance in natural microbial communities. Science 320, 1047–1050 (2008).
Tyson, G. W. & Banfield, J. F. Rapidly evolving CRISPRs implicated in acquired resistance of microorganisms to viruses. Environ. Microbiol. 10, 200–207 (2008).
Emerson, J. B. et al. Virus–host and CRISPR dynamics in archaea-dominated hypersaline Lake Tyrrell, Victoria, Australia. Archaea 2013, 370871 (2013).
Delaney, N. F. et al. Ultrafast evolution and loss of CRISPRs following a host shift in a novel wildlife pathogen, Mycoplasma gallisepticum. PLoS Genet. 8, e1002511 (2012).
Diez-Villasenor, C., Almendros, C., Garcia-Martinez, J. & Mojica, F. J. Diversity of CRISPR loci in Escherichia coli. Microbiology 156, 1351–1361 (2010).
Pougach, K. et al. Transcription, processing and function of CRISPR cassettes in Escherichia coli. Mol. Microbiol. 77, 1367–1379 (2010).
Westra, E. R. et al. H-NS-mediated repression of CRISPR-based immunity in Escherichia coli K12 can be relieved by the transcription activator LeuO. Mol. Microbiol. 77, 1380–1393 (2010).
Pul, U. et al. Identification and characterization of E. coli CRISPR–Cas promoters and their silencing by H-NS. Mol. Microbiol. 75, 1495–1512 (2010).
Brouns, S. J. et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 321, 960–964 (2008).
Touchon, M. et al. CRISPR distribution within the Escherichia coli species is not suggestive of immunity-associated diversifying selection. J. Bacteriol. 193, 2460–2467 (2011).
Touchon, M. & Rocha, E. P. The small, slow and specialized CRISPR and anti-CRISPR of Escherichia and Salmonella. PLoS ONE 5, e11126 (2010).
Touchon, M. et al. Antibiotic resistance plasmids spread among natural isolates of Escherichia coli in spite of CRISPR elements. Microbiology 158, 2997–3004 (2012).
Ketting, R. F. The many faces of RNAi. Dev. Cell 20, 148–161 (2011).
Kaiser, D., Robinson, M. & Kroos, L. Myxobacteria, polarity, and multicellular morphogenesis. Cold Spring Harb. Perspect. Biol. 2, a000380 (2010).
Thony-Meyer, L. & Kaiser, D. devRS, an autoregulated and essential genetic locus for fruiting body development in Myxococcus xanthus. J. Bacteriol. 175, 7450–7462 (1993).
Boysen, A., Ellehauge, E., Julien, B. & Sogaard-Andersen, L. The DevT protein stimulates synthesis of FruA, a signal transduction protein required for fruiting body morphogenesis in Myxococcus xanthus. J. Bacteriol. 184, 1540–1546 (2002).
Viswanathan, P., Murphy, K., Julien, B., Garza, A. G. & Kroos, L. Regulation of dev, an operon that includes genes essential for Myxococcus xanthus development and CRISPR-associated genes and repeats. J. Bacteriol. 189, 3738–3750 (2007).
Kroos, L. & Kaiser, D. Expression of many developmentally regulated genes in Myxococcus depends on a sequence of cell interactions. Genes Dev. 1, 840–854 (1987).
Kroos, L., Kuspa, A. & Kaiser, D. A global analysis of developmentally regulated genes in Myxococcus xanthus. Dev. Biol. 117, 252–266 (1986).
Russo-Marie, F., Roederer, M., Sager, B., Herzenberg, L. A. & Kaiser, D. Beta-galactosidase activity in single differentiating bacterial cells. Proc. Natl Acad. Sci. USA 90, 8194–8198 (1993).
Julien, B., Kaiser, A.D. & Garza, A. Spatial control of cell differentiation in Myxococcus xanthus. Proc. Natl Acad. Sci. USA 97, 9098–9103 (2000).
Louwen, R. et al. A novel link between Campylobacter jejuni bacteriophage defence, virulence and Guillain-Barre syndrome. Eur. J. Clin. Microbiol. Infect. Dis. 32, 207–226 (2013).
Sampson, T. R., Saroj, S. D., Llewellyn, A. C., Tzeng, Y. L. & Weiss, D. S. A. CRISPR/Cas system mediates bacterial innate immune evasion and virulence. Nature 497, 254–257 (2013).
Deltcheva, E. et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 471, 602–607 (2011).
Chylinski, K., Le Rhun, A. & Charpentier, E. The tracrRNA and Cas9 families of type II CRISPR–Cas immunity systems. RNA Biol. 10, 726–737 (2013).
Gunderson, F. F. & Cianciotto, N. P. The CRISPR-associated gene cas2 of Legionella pneumophila is required for intracellular infection of amoebae. mBio 4, e00074–13 (2013).
Beloglazova, N. et al. A novel family of sequence-specific endoribonucleases associated with the clustered regularly interspaced short palindromic repeats. J. Biol. Chem. 283, 20361–20371 (2008).
Toledo-Arana, A. et al. The Listeria transcriptional landscape from saprophytism to virulence. Nature 459, 950–956 (2009).
Mandin, P., Repoila, F., Vergassola, M., Geissmann, T. & Cossart, P. Identification of new noncoding RNAs in Listeria monocytogenes and prediction of mRNA targets. Nucleic Acids Res. 35, 962–974 (2007).
Sesto, N. et al. A PNPase dependent CRISPR system in Listeria. PLoS Genet. 10, e1004065 (2014).
Vercoe, R. B. et al. Cytotoxic chromosomal targeting by CRISPR/Cas systems can reshape bacterial genomes and expel or remodel pathogenicity islands. PLoS Genet. 9, e1003454 (2013).
Edgar, R. & Qimron, U. The Escherichia coli CRISPR system protects from λ lysogenization, lysogens, and prophage induction. J. Bacteriol. 192, 6291–6294 (2010).
Semenova, E. et al. Interference by clustered regularly interspaced short palindromic repeat (CRISPR) RNA is governed by a seed sequence. Proc. Natl Acad. Sci. USA 108, 10098–10103 (2011).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Cady, K. C. & O'Toole, G. A. Non-identity-mediated CRISPR–bacteriophage interaction mediated via the Csy and Cas3 proteins. J. Bacteriol. 193, 3433–3445 (2011).
Zegans, M. E. et al. Interaction between bacteriophage DMS3 and host CRISPR region inhibits group behaviors of Pseudomonas aeruginosa. J. Bacteriol. 191, 210–219 (2009).
Cady, K. C., Bondy-Denomy, J., Heussler, G. E., Davidson, A. R. & O'Toole, G. A. The CRISPR/Cas adaptive immune system of Pseudomonas aeruginosa mediates resistance to naturally occurring and engineered phages. J. Bacteriol. 194, 5728–5738 (2012).
Hale, C. R. et al. RNA-guided RNA cleavage by a CRISPR RNA–Cas protein complex. Cell 139, 945–956 (2009).
Staals, R. H. et al. Structure and activity of the RNA-targeting type III-B CRISPR–Cas complex of Thermus thermophilus. Mol. Cell 52, 135–145 (2013).
Zhang, J. et al. Structure and mechanism of the CMR complex for CRISPR-mediated antiviral immunity. Mol. Cell 45, 303–313 (2012).
Hale, C. R. et al. Essential features and rational design of CRISPR RNAs that function with the Cas RAMP module complex to cleave RNAs. Mol. Cell 45, 292–302 (2012).
Zebec, Z., Manica, A., Zhang, J., White, M.F. & Schleper, C. CRISPR-mediated targeted mRNA degradation in the archaeon Sulfolobus solfataricus. Nucleic Acids Res. http://dx.doi.org/10.1093/nar/gku161 (2014).
Stern, A., Keren, L., Wurtzel, O., Amitai, G. & Sorek, R. Self-targeting by CRISPR: gene regulation or autoimmunity? Trends Genet. 26, 335–340 (2010).
Marraffini, L. A. & Sontheimer, E. J. Self versus non-self discrimination during CRISPR RNA-directed immunity. Nature 463, 568–571 (2010).
Sashital, D. G., Wiedenheft, B. & Doudna, J. A. Mechanism of foreign DNA selection in a bacterial adaptive immune system. Mol. Cell 46, 606–615 (2012).
Westra, E. R. et al. Type I-E CRISPR–Cas systems discriminate target from non-target DNA through base pairing-independent PAM recognition. PLoS Genet. 9, e1003742 (2013).
Brodt, A., Lurie-Weinberger, M. N. & Gophna, U. CRISPR loci reveal networks of gene exchange in archaea. Biol. Direct 6, 65 (2011).
Pleckaityte, M., Zilnyte, M. & Zvirbliene, A. Insights into the CRISPR/Cas system of Gardnerella vaginalis. BMC Microbiol. 12, 301 (2012).
Yosef, I., Goren, M. G. & Qimron, U. Proteins and DNA elements essential for the CRISPR adaptation process in Escherichia coli. Nucleic Acids Res. 40, 5569–5576 (2012).
DeBoy, R. T., Mongodin, E. F., Emerson, J. B. & Nelson, K. E. Chromosome evolution in the Thermotogales: large-scale inversions and strain diversification of CRISPR sequences. J. Bacteriol. 188, 2364–2374 (2006).
Riehle, M. M., Bennett, A. F. & Long, A. D. Genetic architecture of thermal adaptation in Escherichia coli. Proc. Natl Acad. Sci. USA 98, 525–530 (2001).
Aklujkar, M. & Lovley, D. R. Interference with histidyl-tRNA synthetase by a CRISPR spacer sequence as a factor in the evolution of Pelobacter carbinolicus. BMC Evol. Biol. 10, 230 (2010).
Jorth, P. & Whiteley, M. An evolutionary link between natural transformation and CRISPR adaptive immunity. mBio 3, e00309–12 (2012).
Babu, M. et al. A dual function of the CRISPR–Cas system in bacterial antivirus immunity and DNA repair. Mol. Microbiol. 79, 484–502 (2011).
Wiedenheft, B. et al. Structural basis for DNase activity of a conserved protein implicated in CRISPR-mediated genome defense. Structure 17, 904–912 (2009).
Mojica, F. J., Ferrer, C., Juez, G. & Rodriguez-Valera, F. Long stretches of short tandem repeats are present in the largest replicons of the Archaea Haloferax mediterranei and Haloferax volcanii and could be involved in replicon partitioning. Mol. Microbiol. 17, 85–93 (1995).
Makarova, K. S., Aravind, L., Grishin, N. V., Rogozin, I. B. & Koonin, E. V. A. DNA repair system specific for thermophilic archaea and bacteria predicted by genomic context analysis. Nucleic Acids Res. 30, 482–496 (2002).
Williams, E., Lowe, T. M., Savas, J. & DiRuggiero, J. Microarray analysis of the hyperthermophilic archaeon Pyrococcus furiosus exposed to gamma irradiation. Extremophiles 11, 19–29 (2007).
Godde, J. S. & Bickerton, A. The repetitive DNA elements called CRISPRs and their associated genes: evidence of horizontal transfer among prokaryotes. J. Mol. Evol. 62, 718–729 (2006).
Minot, S. et al. Rapid evolution of the human gut virome. Proc. Natl Acad. Sci. USA 110, 12450–12455 (2013).
Minot, S. et al. The human gut virome: inter-individual variation and dynamic response to diet. Genome Res. 21, 1616–1625 (2011).
Sebaihia, M. et al. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nature Genet. 38, 779–786 (2006).
Seed, K. D., Lazinski, D. W., Calderwood, S. B. & Camilli, A. A bacteriophage encodes its own CRISPR/Cas adaptive response to evade host innate immunity. Nature 494, 489–491 (2013).
Novick, R. P., Christie, G. E. & Penades, J. R. The phage-related chromosomal islands of Gram-positive bacteria. Nature Rev. Microbiol. 8, 541–551 (2010).
Makarova, K. S., Anantharaman, V., Aravind, L. & Koonin, E. V. Live virus-free or die: coupling of antivirus immunity and programmed suicide or dormancy in prokaryotes. Biol. Direct 7, 40 (2012).
Cook, G. M. et al. Ribonucleases in bacterial toxin–antitoxin systems. Biochim. Biophys. Acta 1829, 523–531 (2013).
Fineran, P. C. et al. The phage abortive infection system, ToxIN, functions as a protein–RNA toxin–antitoxin pair. Proc. Natl Acad. Sci. USA 106, 894–899 (2009).
Blower, T. R. et al. A processed noncoding RNA regulates an altruistic bacterial antiviral system. Nature Struct. Mol. Biol. 18, 185–190 (2011).
Kwon, A. R. et al. Structural and biochemical characterization of HP0315 from Helicobacter pylori as a VapD protein with an endoribonuclease activity. Nucleic Acids Res. 40, 4216–4228 (2012).
Daines, D. A., Jarisch, J. & Smith, A. L. Identification and characterization of a nontypeable Haemophilus influenzae putative toxin–antitoxin locus. BMC Microbiol. 4, 30 (2004).
Richter, C., Gristwood, T., Clulow, J. S. & Fineran, P. C. In vivo protein interactions and complex formation in the Pectobacterium atrosepticum subtype I-F CRISPR/Cas system. PLoS ONE 7, e49549 (2012).
Nam, K. H. et al. Double-stranded endonuclease activity in Bacillus halodurans clustered regularly interspaced short palindromic repeats (CRISPR)-associated Cas2 protein. J. Biol. Chem. 287, 35943–35952 (2012).
Samai, P., Smith, P. & Shuman, S. Structure of a CRISPR-associated protein Cas2 from Desulfovibrio vulgaris. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 66, 1552–1556 (2010).
Gardner, A. Adaptation as organism design. Biol. Lett. 5, 861–864 (2009).
Fiegna, F. & Velicer, G. J. Competitive fates of bacterial social parasites: persistence and self-induced extinction of Myxococcus xanthus cheaters. Proc. Biol. Sci. 270, 1527–1534 (2003).
Sampson, T. R. & Weiss, D. S. Degeneration of a CRISPR/Cas system and its regulatory target during the evolution of a pathogen. RNA Biol. 10, 1618–1622 (2013).
Schunder, E., Rydzewski, K., Grunow, R. & Heuner, K. First indication for a functional CRISPR/Cas system in Francisella tularensis. Int. J. Med. Microbiol. 303, 51–60 (2013).
Haft, D. H., Selengut, J., Mongodin, E. F. & Nelson, K. E. A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Comput. Biol. 1, e60 (2005).
Karatan, E. & Watnick, P. Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol. Mol. Biol. Rev. 73, 310–347 (2009).
Mikkelsen, H., Sivaneson, M. & Filloux, A. Key two-component regulatory systems that control biofilm formation in Pseudomonas aeruginosa. Environ. Microbiol. 13, 1666–1681 (2011).
Bondy-Denomy, J., Pawluk, A., Maxwell, K. L. & Davidson, A. R. Bacteriophage genes that inactivate the CRISPR/Cas bacterial immune system. Nature 493, 429–432 (2013).
Severinov, K. CRISPR–Cas: outstanding questions remain: comment on “Diversity, evolution & therapeutic applications of small RNAs in prokaryotic and eukaryotic immune systems” by Edwin L. Cooper and Nicola Overstreet. Phys. Life Rev. 11, 146–148 (2013).
Vos, M. Why do bacteria engage in homologous recombination? Trends Microbiol. 17, 226–232 (2009).
Bikard, D., Hatoum-Aslan, A., Mucida, D. & Marraffini, L. A. CRISPR interference can prevent natural transformation and virulence acquisition during in vivo bacterial infection. Cell Host Microbe 12, 177–186 (2012).
Palmer, K. L. & Gilmore, M. S. Multidrug-resistant enterococci lack CRISPR–Cas. mBio 1, e00227–10 (2010).
Carte, J., Wang, R., Li, H., Terns, R. M. & Terns, M. P. Cas6 is an endoribonuclease that generates guide RNAs for invader defense in prokaryotes. Genes Dev. 22, 3489–3496 (2008).
Hale, C., Kleppe, K., Terns, R. M. & Terns, M. P. Prokaryotic silencing (psi)RNAs in Pyrococcus furiosus. RNA 14, 2572–2579 (2008).
Marraffini, L. A. & Sontheimer, E. J. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science 322, 1843–1845 (2008).
Garneau, J. E. et al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 468, 67–71 (2010).
Westra, E. R. et al. CRISPR immunity relies on the consecutive binding and degradation of negatively supercoiled invader DNA by Cascade and Cas3. Mol. Cell 46, 595–605 (2012).
Reeks, J., Naismith, J. H. & White, M. F. CRISPR interference: a structural perspective. Biochem. J. 453, 155–166 (2013).
Jore, M. M. et al. Structural basis for CRISPR RNA-guided DNA recognition by Cascade. Nature Struct. Mol. Biol. 18, 529–536 (2011).
Rouillon, C. et al. Structure of the CRISPR interference complex CSM reveals key similarities with Cascade. Mol. Cell 52, 124–134 (2013).
Hatoum-Aslan, A., Maniv, I., Samai, P. & Marraffini, L. A. Genetic characterization of anti-plasmid immunity by a type III-A CRISPR–Cas system. J. Bacteriol. 196, 310–317 (2013).
Sorek, R., Lawrence, C. M. & Wiedenheft, B. CRISPR-mediated adaptive immune systems in bacteria and archaea. Annu. Rev. Biochem. 82, 237–266 (2013).
Zusman, D. R., Scott, A. E., Yang, Z. & Kirby, J. R. Chemosensory pathways, motility and development in Myxococcus xanthus. Nature Rev. Microbiol. 5, 862–872 (2007).
Acknowledgements
E.R.W. received funding from the People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme (FP7/2007-2013) under Research Executive Agency (REA) grant agreement number 327606. A.B. is supported by a UK Royal Society Wolfson Research Merit Award. P.C.F. is supported by a Rutherford Discovery Fellowship from the Royal Society of New Zealand.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (figure)
The partial complementarity between the scaRNA, tracrRNA and blp transcript is shown in detail, which is proposed to facilitate complex formation and subsequent degradation of the blp transcript. (PDF 206 kb)
Rights and permissions
About this article
Cite this article
Westra, E., Buckling, A. & Fineran, P. CRISPR–Cas systems: beyond adaptive immunity. Nat Rev Microbiol 12, 317–326 (2014). https://doi.org/10.1038/nrmicro3241
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro3241
This article is cited by
-
Crop bioengineering via gene editing: reshaping the future of agriculture
Plant Cell Reports (2024)
-
Importance of mobile genetic element immunity in numerically abundant Trichodesmium clades
ISME Communications (2023)
-
Genomic Analyses of Pediococcus pentosaceus ST65ACC, a Bacteriocinogenic Strain Isolated from Artisanal Raw-Milk Cheese
Probiotics and Antimicrobial Proteins (2023)
-
Probiotic Strain Limosilactobacillus reuteri 29B is Proven Safe and Exhibits Potential Probiotic Traits in a Murine Vaginal Model
Probiotics and Antimicrobial Proteins (2023)
-
Characterization and diversity of CRISPR/Cas systems in Klebsiella oxytoca
Molecular Genetics and Genomics (2023)