Key Points
-
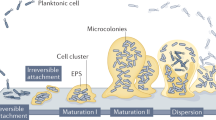
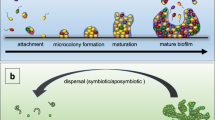
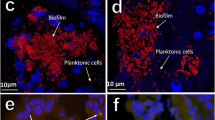
Biofilms are clusters of microorganisms that stick to non-biological surfaces, such as rocks in a stream, as well as to surfaces on plants (roots) or in animals (epithelium).
-
These clusters are often encased in an outer polymer layer that can be produced by the microorganism or by the defensive mechanisms of the colonized host.
-
Biofilm formation is astonishingly widespread in nature and appears very early in the fossil record. Biofilm development also occurs in a vastly diverse range of microorganisms, including those that have changed little over of time. So, biofilm formation seems to be an ancient and fundamental part of the life cycles of many microorganisms and essential for survival in diverse environments.
-
Biofilm formation represents a protected mode of growth that not only allows cells to survive in hostile environments, but also to colonize new niches by dispersal of microorganisms from the microbial clusters.
-
Biofilms are an important, but incompletely understood, form of growth and survival for many bacteria. Recent evidence reveals that biofilms are structurally complex, dynamic systems that have both the characteristics of primitive multicellularly organized organisms and complex ecosystems.
-
In this review, the implications of survival and dispersal mechanisms are discussed in the context of both the natural environment and infectious diseases.
Abstract
Biofilms — matrix-enclosed microbial accretions that adhere to biological or non-biological surfaces — represent a significant and incompletely understood mode of growth for bacteria. Biofilm formation appears early in the fossil record (∼3.25 billion years ago) and is common throughout a diverse range of organisms in both the Archaea and Bacteria lineages, including the 'living fossils' in the most deeply dividing branches of the phylogenetic tree. It is evident that biofilm formation is an ancient and integral component of the prokaryotic life cycle, and is a key factor for survival in diverse environments. Recent advances show that biofilms are structurally complex, dynamic systems with attributes of both primordial multicellular organisms and multifaceted ecosystems. Biofilm formation represents a protected mode of growth that allows cells to survive in hostile environments and also disperse to colonize new niches. The implications of these survival and propagative mechanisms in the context of both the natural environment and infectious diseases are discussed in this review.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zobel, C. E. The effect of solid surfaces upon bacterial activity. J. Bacteriol. 46, 39–56 (1943).
Doyle, R. (ed.). Microbial growth in biofilms, part A: developmental and molecular biological aspects. Methods Enzymol. 336 (2001).
Doyle, R. (ed.) Microbial growth in biofilms, part B: special environments and physiochemical aspects. Methods Enzymol. 337 (2001).
Westall, F. et al. Early Archean fossil bacteria and biofilms in hydrothermally-influenced sediments from the Barberton greenstone belt, South Africa. Precambrian Res. 106, 93–116 (2001).
Rasmussen, B. Filamentous microfossils in a 3,235-million-year-old volcanogenic massive sulphide deposit. Nature 405, 676–679 (2000).
Reysenbach, A. L. & Cady, S. L. Microbiology of ancient and modern hydrothermal systems Trends Microbiol. 9, 79–86 (2001).
Taylor, C. D., Wirsen, C. O. & Gaill, F. Rapid microbial production of filamentous sulfur mats at hydrothermal vents. Appl. Environ. Microbiol. 65, 2253–2255 (1999).
Jahnke, L. L. et al. Signature lipids and stable carbon isotope analyses of octopus spring hyperthermophilic communities compared with those of aquificales representatives. Appl. Environ. Microbiol. 67, 5179–5189 (2001).
Reysenbach, A. L., Ehringer, M. & Hershberger, K. Microbial diversity at 83 degrees C in Calcite Springs, Yellowstone National Park: another environment where the Aquificales and 'Korarchaeota' coexist. Extremophiles 4, 61–67 (2000).
Stoodley, P., Sauer, K., Davies, D. G. & Costerton, J. W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56, 187–209 (2002).
Baty, A. M., Eastburn, C. C., Techkarnjanaruk, S., Goodman, A. E. & Geesey, G. G. Spatial and temporal variations in chitinolytic gene expression and bacterial biomass production during chitin degradation. Appl. Environ. Microbiol. 66, 3574–3585 (2000). Discovered that there could be a 'division of labour' in bacterial biofilm populations such that a subset that remained attached could degrade the chitin substratum and provide nutrients for a detached, planktonic subset.
Edwards, K. J., Bond, P. L., Gihring, T. M. & Banfield, J. F. An archaeal iron-oxidizing extreme acidophile important in acid mine drainage. Science. 287, 1731–1732 (2000).
Klausen, M. et al. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol Microbiol. 48, 1511–1524 (2003).
Stoodley, P., Dodds, I., Boyle, J. D. & Lappin-Scott, H. M. Influence of hydrodynamics and nutrients on biofilm structure. J. Appl. Microbiol. 85, 19S–28S (1999).
Sauer, K., Camper, A. K., Ehrlich, G. D., Costerton, J. W. & Davies, D. G. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184, 1140–1154 (2002). Linked structural development with protein expression in Pseudomonas biofilms thereby demonstrating that biofilms can have regulated 'life-cycles'.
Ghigo, J.-M. Are there biofilm-specific physiological pathways beyond a reasonable doubt? Res. Microbiol. 154, 1–8 (2003).
van Loosdrecht, M. C., Heijnen, J. J., Eberl, H., Kreft, J. & Picioreanu, C. Mathematical modelling of biofilm structures. Antonie Van Leeuwenhoek. 81, 245–256 (2002).
Lawrence, J. R., Korber, D. R., Hoyle, B. D., Costerton, J. W. & Caldwell, D. E. Optical sectioning of microbial biofilms. J. Bacteriol. 173, 6558–6567 (1991).
deBeer, D., Stoodley, P., Roe, F. & Lewandowski, Z. Effects of biofilm structures on oxygen distribution and mass transport. Biotechnol. Bioeng. 43, 1131–1138 (1994).
Stoodley, P., de Beer, D. & Lewandowski, Z. Liquid flow in biofilm systems. Appl. Environ. Microbiol. 60, 2711–2716 (1994).
Pratt, L. A. & Kolter, R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 30, 285–293 (1998).
Reisner, A., Haagensen, J. A., Schembri, M. A., Zechner, E. L. & Molin, S. Development and maturation of Escherichia coli K-12 biofilms. Mol. Microbiol. 48, 933–946 (2003).
Tremoulet, F., Duche, O., Namane, A., Martinie, B. & Labadie, J. C. A proteomic study of Escherichia coli O157:H7 NCTC 12900 cultivated in biofilm or in planktonic growth mode. FEMS Microbiol. Lett. 215, 7–14 (2002).
Watnick, P. I. & Kolter, R. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol. Microbiol. 34, 586–595 (1999).
Kaiser, D. Coupling cell movement to multicellular development in myxobacteria. Nature Rev. Microbiol. 1, 45–54 (2003)
Fux, C. A., Stoodley, P., Hall-Stoodley, L. & Costerton, W. J. Bacterial biofilms—a diagnostic and therapeutic challenge. Expert Rev. Anti-Infective Ther. 1, 667–683 (2003).
Hall-Stoodley, L., Keevil, C. W. & Lappin-Scott, H. M. Mycobacterium fortuitum and Mycobacterium chelonae form biofilms under high and low nutrient conditions. J. Appl. Microbiol. 85, S60–S69 (1999).
Bracco, E. et al. Cell signaling and adhesion in phagocytosis and early development of Dictyostelium. Int. J. Dev. Biol. 4, 733–742 (2000).
Hall-Stoodley, L. & Stoodley, P. Development regulation of microbial biofilms. Curr. Opin. Biotech. 13, 228–233 (2002).
Kjelleberg, S. & Molin, S. Is there a role for quorum sensing signals in bacterial biofilms? Curr. Opin. Microbiol. 5, 254–258 (2002). Discusses the relative contribution of various environmental factors (such as flow and nutrients) and genetic factors (cell signalling) on biofilm structure.
Hunt, S. M., Hamilton, M. A., Sears, J. T., Harkin, G. & Reno, J. A computer investigation of chemically mediated detachment in bacterial biofilms. Microbiology 149, 1155–1163 (2003).
Caiazza, N. C. & O'Toole, G. A. Alpha-toxin is required for biofilm formation by Staphylococcus aureus. J. Bacteriol. 185, 3214–3217 (2003).
Cucarella, C. et al. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 183, 2888–2928 (2001).
Cramton, S. E., Gerke, C., Schnell, N. F., Nichols, W. W. & Cotz, F. The intercellular adhesion (ica) locas is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67, 5427–5433 (1999).
Hamon, M. A. & Lazazzera, B. A. The sporulation transcription factor Spo0A is required for biofilm development in Bacillus subtilis. Mol. Microbiol. 42, 1199–1209 (2001).
Froeliger, E. H. & Fives-Taylor, P. Streptococcus parasanguis fimbria-associated adhesion. Fap1 is required for biofilm formation. Infect. Immun. 69, 2512–2519 (2001).
Gav'n, R. et al. Lateral flagella of Aeromonas species are essential for epithelial cell adherence and biofilm formation. Mol. Microbiol. 43, 383–397 (2002).
Whitchurch, C. B., Tolker-Nielsen, T., Ragas, P. C. & Mattick, J. S. Extracellular DNA required for bacterial biofilm formation. Science 295, 1487 (2002).
Valle, J. et al. SarA and not sigmaB is essential for biofilm development by Staphylococcus aureus. Mol. Microbiol. 48, 1075–1087 (2003).
Davies, D. G. et al. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280, 295–298 (1998). Demonstrated that cell–cell communication molecules, associated with the production of virulence factors, have a role in the structure of Pseudomonas biofilms, opening the concept that biofilm structure was genetically regulated.
Heydorn, A. et al. Statistical analysis of Pseudomonas aeruginosa biofilm development: impact of mutations in genes involved in twitching motility, cell-to-cell signaling, and stationary-phase sigma factor expression. Appl. Environ. Microbiol. 68, 2008–2017 (2002).
Purevdorj, B., Costerton, J. W. & Stoodley, P. Influence of hydrodynamics and cell signaling on the structure and behavior of Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 68, 4457–4464 (2002). Demonstrated that environmental factors (in this case, flow) can 'override' cell–cell communications as a principal determinant of biofilm structure, illustrating that biofilm development is a multifactorial process influenced by both environmental and genetic factors.
Stoodley, P., Jørgensen, F., Williams, P. & Lappin-Scott, H. M. in Biofilms: The Good, the Bad, and the Ugly (eds Bayston, R., Brading, M., Gilbert, P., Walker, J. & Wimpenny, J. W. T.) 323–330 (BioLine, Cardiff, UK, 1999)
Stoodley, P., Cargo, R., Rupp, C. J., Wilson, S., & Klapper, I. Biofilm mechanics and shear-induced deformation and detachment. J. Industrial Microbiol. Biotech. 29, 361–368 (2002).
Boyd, A. & Chakrabarty, A. M. Role of alginate lyase in cell detachment of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 60, 2355–2359 (1994).
Kaplan, J. B., Ragunath, C., Ramasubbu, N. & Fine, D. H. Detachment of Actinobacillus actinomycetemcomitans biofilm cells by an endogenous β-hexosaminidase activity. J. Bacteriol. 185, 4693–4698 (2003).
Lee, S. F., Li, Y. H. & Bowden, G. H. Detachment of Streptococcus mutans biofilm cells by an endogenous enzymatic activity. Infect. Immun. 64, 1035–1038 (1996).
Piriou, P., Dukan, S., Levi, Y. & Jarrige, P. A. Prevention of bacterial growth in drinking water distribution systems. Water Sci. Technol. 35, 283–287 (1997).
Zottola, E. A. & Sasahara, K. C. Microbial biofilms in the food industry should they be a concern? Int. J. Food Microbiol. 23, 125–148 (1994).
Lowy, F. D. Staphylococcus aureus infections. N. Engl. J. Med. 339, 520–532 (1998).
Pankhurst, C. L., Johnson, N. W. & Woods, R. G. Microbial contamination of dental unit waterlines: the scientific argument. Int. Dent. J. 48, 359–368 (1998).
Raad, I. I. Catheter-related septicemia: risk reduction. Infect. Med. 13, 807–812, 815–816, 823 (1996).
Purevdorj, B. & Stoodley, P. in Microbial Biofilms. (eds Ghannoum, M. A. and O'Toole, G.) (ASM Press, Washington DC, USA, in the press).
Tolker-Nielsen, T. et al. Development and dynamics of Pseudomonas sp. biofilms. J. Bacteriol. 182, 6482–6489 (2000).
Webb, J. S. et al. Cell death in Pseudomonas aeruginosa biofilm development. J. Bacteriol. 185, 4585–4592 (2003).
Spoering, A. L. & Lewis, K. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J. Bacteriol. 183, 6746–6751 (2001). Links the antibiotic resistance of biofilms to the stationary phase physiology of cells within the biofilms and the presence of a small phenotypically distinct 'persister' population.
Hanlon, G. W., Denyer, S. P., Olliff, C. J. & Ibrahim, L. J. Reduction in exopolysaccharide viscosity as an aid to bacteriophage penetration through Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 67, 2746–2753 (2001).
Kaplan, J. B., Meyenhofer, M. F. & Fine, D. H. Biofilm growth and detachment of Actinobacillus actinomycetemcomitans. J. Bacteriol. 185, 1399–1404 (2003).
Kaplan, J. B. & Fine, D. H. Biofilm dispersal of Neisseria subflava and other phylogenetically diverse oral bacteria. Appl. Environ. Microbiol. 68, 4943–4950 (2002).
Stoodley, P. et al. Growth and detachment of cell clusters from mature mixed species biofilms. Appl. Environ. Microbiol. 67, 5608–5613 (2001).
Mattick, J. S. Type IV pili and twitching motility. Annu. Rev. Microbiol. 56, 289–314 (2002).
Stoodley, P., Lewandowski, Z., Boyle, J. D. & Lappin-Scott, H. M. The formation of migratory ripples in a mixed species bacterial biofilm growing in turbulent flow. Environ. Microbiol. 1, 447–457 (1999).
Inglis, T. J. J. Evidence for dynamic phenomena in residual trachael tube biofilm. Br. J. Anaesth. 70, 22–24 (1993).
Stewart, P. S. & Costerton, J. W. Antibiotic resistance of bacteria in biofilms. Lancet. 358, 135–138 (2001).
Klapper, I., Rupp, C. J., Cargo, R., Purevdorj, B. & Stoodley, P. A viscoelastic fluid description of bacterial biofilm material properties. Biotech. Bioeng. 80, 289–296 (2002).
Korstgens, V., Flemming, H. C., Wingender, J. & Borchard, W. Uniaxial compression measurement device for investigation of the mechanical stability of biofilms. J. Microbiol. Meth. 46, 9–17 (2001).
Towler, B. W., Rupp, C. J., Cunningham, A. B. & Stoodley, P. Viscoelastic properties of a mixed culture biofilm from rheometer creep analysis. Biofouling 19, 279–285 (2003).
Vinogradov, A. M., Winston., M., Rupp., C. J. & Stoodley, P. Rheology of biofilms formed from the dental plaque pathogen Streptococcus mutans. Biofilms (in the press).
Espeland, E. M. & Wetzel, R. G. Complexation, stabilization, and UV photolysis of extracellular and surface-bound glucosidase and alkaline phosphatase: implications for biofilm microbiota. Microb. Ecol. 42, 572–585 (2001).
Teitzel, G. M. & Parsek, M. R. Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl. Environ. Microbiol. 69, 2313–2320 (2003).
McNeill, K. & Hamilton, I. R. Acid tolerance response of biofilm cells of Streptococcus mutans. FEMS Microbiol. Lett. 221, 25–30 (2003).
Le Magrex-Debar, E., Lemoine, J., Gelle, M. P., Jacquelin, L. F. & Choisy, C. Evaluation of biohazards in dehydrated biofilms on foodstuff packaging. Int. J. Food Microbiol. 55, 239–234 (2000).
Leid, J. G., Shirtliff, M. E., Costerton, J. W. & Stoodley, P. Human leukocytes adhere, penetrate, and respond to Staphylococcus aureus biofilms. Infect. Immun. 70, 6339–6345 (2002).
Gilbert, P., Allison, D. G. & McBain, A. J. Biofilms in vitro and in vivo: do singular mechanisms imply cross-resistance? J. Appl. Microbiol. 92, S98–S110 (2002).
Mah, T. F. & O'Toole, G. A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9, 34–39 (2001).
Dibdin, G. H., Assinder, S. J., Nichols, W. W. & Lambert, P. A. Mathematical model of β-lactam penetration into a biofilm of Pseudomonas aeruginosa while undergoing simultaneous inactivation by released β-lactamases. J. Antimicrob. Chemother. 38, 757–769 (1996).
Anderl, J. N., Zahller, J., Roe, F. & Stewart, P. S. Role of nutrient limitation and stationary-phase existence in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 47, 1251–1256 (2003).
Walters, M. C., Roe, F., Bugnicourt, A., Franklin, M. J. & Stewart, P. S. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob. Agents Chemother. 47, 317–323 (2003).
Suci, P. A. & Tyler, B. J. A method for discrimination of subpopulations of Candida albicans biofilm cells that exhibit relative levels of phenotypic resistance to chlorhexidine. J. Microbiol. Methods 53, 313–325 (2003).
Parsek, M. R. & Singh, P. K. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 57, 677–701 (2003). Discusses biofilm pathogenesis and defines some clinical criteria for classifying infections with a biofilm aetiology.
Donlan, R. M. & Costerton, J. W. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15, 167–193 (2002). An excellent, comprehensive review of medically relevant biofilms.
Gotz, F. Staphylococcus and biofilms. Mol. Microbiol. 43, 1367–1378 (2002). An excellent review of staphylococcal biofilms and the molecular mechanisms of adhesion and biofilm development in staphylococci.
Peters, G., Locci, R. & Pulverer, G. Microbial colonization of prosthetic devices. II. Scanning electron microscopy of naturally infected intravenous catheters. Zentralb. Bacteriol. Mikrobiol. Hyg. 173, 293–299 (1981).
Christensen, G. D. Simpson, W. A., Bisno, A. L. & Beachey, E. H. Phenotypic variation of Staphylococcus epidermidis slime production in vitro and in vivo. Infect. Immun. 55, 622–628 (1982).
Marrie, T. J., Nelligan, J. & Costerton, J. W. A scanning and transmission electron microscopic study of and infected endocardial pacemaker lead. Circulation 66, 1339–1341 (1982). A seminal paper showing biofilm formation on a medical device.
von Eiff, C., Heilmann, C., Hermann, M. & Peters, G. Basic aspects of the pathogenesis of staphylococcal polymer-associated infections. Infection 27, S7–S10 (1999).
Akiyama, H., Huh, W. K., Yamasaki, O., Oono, T. & Iwatsuki, K. Confocal laser scanning microscopic observation of glycocalyx production by Staphylococcus aureus in mouse skin: does S. aureus generally produce a biofilm on damaged skin? Br. J. Dermatol. 147, 879–885 (2002).
Mack, D. et al. The intercellular adhesion involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glycosaminoglycan: purification and structural analysis. J. Bacteriol. 178, 175–183 (1996).
Heilmann, C., Hussain, M., Peters, G. & Gotz, F. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 24, 1013–1024 (1997).
Heilmann, C., Gerke, C., Perdreau-Remington, F. & Gotz, F. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect. Immun. 64, 277–282 (1996).
Heilmann, C. et al. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20, 1083–1091 (1996).
Gross, M., Cramton, S. E., Gotz, F. & Peschel, A. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect. Immun. 69, 3423–3426 (2001).
Dunne, W. M. Jr & Burd, E. M. The effects of magnesium, calcium, EDTA and pH on the in vitro adhesion of Staphylococcus epidermidis to plastic. Microbiol. Immunol. 36, 1019–1027 (1992).
Vaudaux, P. E, Lew, D. P. & Waldvogel, F. in Infections Associated with Indwelling Medical Devices. 2nd Edition (eds Bisno, A. L. & Waldvogel, F. A.) (ASM Press, Washington DC, USA, 1994).
Foster, T. J. & Höök, M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6, 484–488 (1998).
Vaudaux, P. E. et al. Use of adhesion defective mutants of Staphylococcus aureus to define the role of specific plasma proteins in promoting adhesion to arteriovenous shunts. Infect. Immun. 63, 585–590 (1995).
Durack, D. T. Experimental bacterial endocarditis. IV. Structure and evolution of very early lesions. J. Pathol. 115, 81–89 (1975).
Höök, E. W. & Sande, M. A. Role of the vegetation in experimental Streptococcus viridans endocarditis. Infect. Immun. 10, 1433–1438 (1974).
Marrie, T. J, Cooper, J. H. & Costerton, J. W. Ultrastructure of cardiac bacterial vegetations on native valves with emphasis on alterations in bacterial morphology following antibiotic treatment. Can. J. Cardiol. 3, 275–280 (1987).
Ramirez-Rhonda, C. H. Adherence of glucan-positive and glucan-negative streptococcal strains to normal and damaged heart valves. J. Clin. Invest. 62, 805–814 (1978).
Fey, P. D. et al. Characterization of the relationship between polysaccharide intercellular adhesin and hemagglutination in Staphylococcus epidermidis. J. Infect. Dis. 179, 1561–1564 (1999).
Shiro, H. et al. Transposon mutants of Staphylococcus epidermidis deficient in elaboration of capsular polysaccharide/adhesin and slime are avirulent in a rabbit model of endocarditis. J. Infect. Dis. 169, 1042–1049 (1994).
Sullam, P. M., Bayer, A. S., Foss, W. M. & Cheung, A. L. Diminished platelet binding in vitro by Staphylococcus aureus is associated with reduced virulence in a rabbit model of infective endocarditis. Infect. Immun. 64, 4915–4921 (1996).
Kuypers, J. M. & Proctor, R. A. Reduced adherence to traumatized rat heart valves by a low-fibronectin-binding mutant of Staphylococcus aureus. Infect. Immun. 57, 2306–2312 (1989).
Moreillon, P. et al. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect. Immun. 63, 4738–4743 (1995).
Que, Y. A. et al. Reassessing the role of Staphylococcus aureus clumping factor and fibronectin-binding protein by expression in Lactococcus lactis. Infect. Immun. 69, 6296–6302 (2001).
Siboo, I. R., Cheung, A. L., Bayer, A. S. & Sullam, P. M. Clumping factor A mediates binding of Staphylococcus aureus to human platelets. Infect. Immun. 69, 3120–3127 (2001).
Joly, V. et al. Value of antibiotic levels in serum and cardiac vegetations for predicting antibacterial effect of ceftriaxone in experimental Escherichia coli endocarditis. Antimicrob. Agents Chemother. 31, 1632–1639 (1987).
Lyczak, J. B., Cannon, C. L. & Pier, G. B. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15, 194–222 (2002).
Koch, C. & Hoiby, N. Pathogenesis of cystic fibrosis. Lancet 341, 1065–1069 (1993).
Govan, J. R. & Deretic, V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60, 539–574 (1996).
Costerton, J. W., Irvin, R. T. & Cheng, K. J. The role of bacterial surface structures in pathogenesis. Crit. Rev. Microbiol. 8, 303–338 (1981).
Costerton, J. W., Lam, J., Lam, K. & Chan, R. The role of the microcolony mode of growth in the pathogenesis of Pseudomonas aeruginosa infections. Rev. Infect. Dis. 5, S867–S873 (1983).
Lam, J., Chan, R., Lam, K., & Costerton, J. W. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun. 28, 546–556 (1980). A seminal paper suggesting Pseudomonas pneumonia in cystic fibrosis is a biofilm infection.
Singh, P. K. et al. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407, 762–764 (2000). Presents several criteria to show that biofilm infections are present in cystic fibrosis.
Potts, S. B., Roegli, V. L. & Spock, A. Immunohistologic quantification of Pseudomonas aeruginosa in the tracheo-bronchial tree from patients with cystic fibrosis. Pediatr. Path. Lab. Med. 15, 707–721 (1995).
Mathee, K. et al. Mucoid conversion of Pseudomonas aeruginosa by hydrogen peroxide: a mechanism for virulence activation in the cystic fibrosis lung. Microbiology 145, 1349–1357 (1999). Links inflammatory responses of the host to the emergence of a virulent P. aeruginosa mucoid phenotype.
Suter, S., Schaad, U. B., Morgenthaler, J. J., Chevallier, I. & Schnebli, H. P. Fibronectin-cleaving activity in bronchial secretions of patients with cystic fibrosis. J. Infect. Dis. 158, 89–100 (1988).
Saiman, L. & Prince, A. Pseudomonas aeruginosa pili bind to asialoGM1 which is increased on the surface of cystic fibrosis epithelial cells. J. Clin. Invest. 92, 1875–1880 (1993).
Roger, P. et al. Fibronectin and α5β1 integrin mediate binding of Pseudomonas aeruginosa to repairing airway epithelium. Eur. Respir. J. 13, 1301–1309 (1999).
Ofek, I., Hasty, D. L. & Doyle, R. J. (eds). Bacterial Adhesion to Animal Cells and Tissues (ASM Press, Washington DC, USA, 2003).
Smith, R. S., Harris, S. G., Phipps, R. & Iglewski, B. The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl) homoserine lactone contributes to virulence and induces inflammation in vivo. J. Bacteriol. 184, 1132–1139 (2002).
Smith, R. S., Kelly, R., Iglewski, B. H. & Phipps, R. P. The Pseudomonas autoinducer N-(3-oxododecanoyl) homoserine lactone induces cyclooxygenase-2 and prostaglandin E2 production in human lung fibroblasts: implications for inflammation. J. Immunol. 169, 2636–2642 (2002).
Worlitzsch, D. et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Invest. 109, 317–325 (2002).
Yoon, S. S. et al. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev. Cell. 3, 593–603 (2002).
Xu, K. D., Stewart, P. S., Xia, F., Huang, C. T. & McFeters, G. A. Spatial physiological heterogeneity in Pseudomonas aeruginosa biofilm is determined by oxygen availability. Appl. Environ. Microbiol. 64, 4035–4039 (1998). Showed that biofilm cells could exhibit a wide range of physiologies from stationary phase to a highly active phase over very small distances (micrometres), due to the heterogeneity in nutrient distribution (in this case, oxygen) resulting from the mass transfer characteristics and the shape of the biofilm microcolonies.
Drenkard, E. & Ausubel, F. M. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 416, 740–743 (2002).
Huang, C. T., Xu, K. D., McFeters, G. A. & Stewart, P. S. Spatial patterns of alkaline phosphatase expression within bacterial colonies and biofilms in response to phosphate starvation. Appl. Environ. Microbiol. 64, 1526–1531 (1998).
deBeer, D. & Stoodley, P. Relation between the structure of an aerobic biofilm and mass transport phenomena. Water Sci. Tech. 32, 11–18 (1995).
Donlan, R. M. et al. in Legionella (eds Marre, R. et al.) 406–410 (ASM Press, Washington DC, USA, 2002).
Heydorn, A. et al. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146, 2395–2407 (2000).
Yang, X., Beyenal, H., Harkin, G. & Lewandowski, Z. Quantifying biofilm structure using image analysis. J. Microbiol. Methods 39, 109–119 (2000).
Acknowledgements
Financial support was provided by the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez
FURTHER INFORMATION
Glossary
- BIOFILM
-
Microbial biofilms are populations of microorganisms that are concentrated at an interface (usually solid–liquid) and typically surrounded by an extracellular polymeric substance (EPS) matrix. Aggregates of cells not attached to a surface are sometimes termed 'flocs' and have many of the same characteristics as biofilms.
- PLANKTONIC CELLS
-
Planktonic (or suspended) cell cultures are those grown primarily as single cells in suspension, either in a chemostat or a shake flask.
- SESSILE
-
In an ecological context, a stationary organism such as a plant or a barnacle. In biofilm microbiology, it is used to distinguish planktonic (free-floating) prokaryotic cells from those attached to surfaces. However, new evidence shows that these 'sessile' cells are often dynamic, at least on the microscopic scale.
- STREAMERS
-
Filamentous biofilm microcolonies that form in flowing water. The streamers are atached to the surface by an upstream 'head', while the downstream 'tail' can oscillate in the current.
- PERIPHYTON
-
An assemblage of organisms attached to and living on submerged solid surfaces in natural environments such as rivers.
- STROMATOLITE
-
Mushroom- and tower-shaped structures formed by layers of cyanobacteria and entrapped sediments that grow in quiescent (calm) saline and hydrothermal waters.
- MASS TRANSFER
-
In the context of biofilms, mass transfer refers to the process by which dissolved and particulate substances (such as nutrients) are moved into and out of the biofilm by the surrounding fluid.
- ADHESION
-
A stable interaction of a cell with respect to a surface. Living cells actively excrete chemicals from their surface to anchor themselves to a substratum. This is referred to as adhesion or attachment.
- TYPE IV PILUS
-
An elongated structure extending from the surface of Gram-negative cells that is independent of flagella and which can retract and pull the cell forward.
- DETACHMENT
-
The loss of single cells or aggregates of cells from the biofilm, usually into an overlying flow of fluid. Detachment can be an active process (dispersal), a passively induced mechanical process (for example, through fluid shear) or a chemical process (by adding agents that 'dissolve' the EPS matrix).
- FLUID SHEAR
-
The mechanical force that is exerted by a fluid as it moves past a surface. Although shear exists throughout the fluid in biofilms, 'shear stress' is usually used in the context of the shear exerted at the solid surface — for example, where the biofilm is growing. The shear stress will tend to 'wash away' the attached biofilm from the surface on which it is growing, and it increases as the flow rate is increased.
- EPS
-
Extracellular polymeric substance. Polymers of varying chemical composition that are excreted by the cells in the biofilm. The EPS is the slime matrix that gives the biofilm stability and helps it to adhere to a surface. Although generally assumed to be primarily composed of polysaccharides, the EPS can also contain proteins and nucleic acids.
- ALGINATE
-
An exopolysaccharide produced by P. aeruginosa, which is believed to contribute to the antibiotic resistance of P. aeruginosa.
- GLIDING OR TWITCHING MOTILITY
-
Movement, predominantly by Gram-negative cells, that is dependent on type IV pili.
- VISCOELASTIC
-
A material that has both elastic (solid-like) and viscous (liquid-like) properties.
- HYDROGELS
-
An extremely hydrated polymer gel. The polymer chain holds many times its weight in trapped water.
- PERITRICHOUS FIMBRIAE
-
The many short extracellular pili appendages that protrude from the surface of some prokaryotic cells. Fimbriae are used for attachment to surfaces.
- QUORUM SENSING
-
A system by which bacteria communicate. Signalling molecules — chemicals similar to pheromones that are produced by an individual bacterium — can affect the behaviour of surrounding bacteria.
Rights and permissions
About this article
Cite this article
Hall-Stoodley, L., Costerton, J. & Stoodley, P. Bacterial biofilms: from the Natural environment to infectious diseases. Nat Rev Microbiol 2, 95–108 (2004). https://doi.org/10.1038/nrmicro821
Issue Date:
DOI: https://doi.org/10.1038/nrmicro821
This article is cited by
-
Combination antimicrobial therapy: in vitro synergistic effect of anti-staphylococcal drug oxacillin with antimicrobial peptide nisin against Staphylococcus epidermidis clinical isolates and Staphylococcus aureus biofilms
Annals of Clinical Microbiology and Antimicrobials (2024)
-
Bacterial c-di-GMP signaling gene affects mussel larval metamorphosis through outer membrane vesicles and lipopolysaccharides
npj Biofilms and Microbiomes (2024)
-
Anti-biofilm properties of laser-synthesized, ultrapure silver–gold-alloy nanoparticles against Staphylococcus aureus
Scientific Reports (2024)
-
Non-biofilm-forming Staphylococcus epidermidis planktonic cell supernatant induces alterations in osteoblast biological function
Scientific Reports (2024)
-
Lactobacillus paracasei R3 Alleviates Tumor Progression in Mice with Colorectal Cancer
Current Microbiology (2024)