Key Points
-
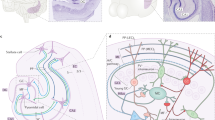
On theoretical grounds, the architecture of CA3 circuits seems to be well adapted for the rapid storage and retrieval of associative memories. This is thought to require plastic changes in the strength of specific synaptic contacts.
-
Dentate gyrus cells provide sparse but powerful synaptic mossy fibre connections to CA3 pyramidal cells, which display a large dynamic range of presynaptic plasticity. This repertoire was recently extended to include postsynaptic plasticity of NMDA receptor-mediated excitatory postsynaptic currents (EPSCs), making these synapses competent for conventional long-term potentiation of AMPA receptor-mediated EPSCs.
-
Local recurrent connectivity gives rise to the CA3 autoassociative network amenable to spike-timing dependent plasticity, which can be facilitated by heterosynaptic interactions.
-
Local GABAergic loops control spike transfer at CA3 connections. GABAergic connectivity is subject to prominent structural and molecular plasticity in relation to memory encoding.
-
Mice impaired in the plasticity of CA3–CA3 or dentate gyrus–CA3 connections show deficits in one-trial memory tasks. Nevertheless, a direct link between memory and functional plasticity of specific excitatory or inhibitory connections is still awaited.
Abstract
The CA3 region of the hippocampus is important for rapid encoding of memory. Computational theories have proposed specific roles in hippocampal function and memory for the sparse inputs from the dentate gyrus to CA3 and for the extended local recurrent connectivity that gives rise to the CA3 autoassociative network. Recently, we have gained considerable new insight into the operation and plasticity of CA3 circuits, including the identification of novel forms of synaptic plasticity and their underlying mechanisms, and structural plasticity in the GABAergic control of CA3 circuits. In addition, experimental links between synaptic plasticity of CA3 circuits and memory are starting to emerge.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kesner, R. P. & Rolls, E. T. A computational theory of hippocampal function, and tests of the theory: new developments. Neurosci. Biobehav. Rev. 48, 92–147 (2015).
McNaughton, B. L. & Morris, R. G. M. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 10, 408–415 (1987).
Rolls, E. T. An attractor network in the hippocampus: theory and neurophysiology. Learn. Mem. 14, 714–731 (2007).
Henze, D. A., Urban, N. N. & Barrionuevo, G. The multifarious hippocampal mossy fiber pathway: a review. Neuroscience 98, 407–427 (2000).
Nicoll, R. A. & Schmitz, D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat. Rev. Neurosci. 6, 863–876 (2005).
Evstratova, A. & Toth, K. Information processing and synaptic plasticity at hippocampal mossy fiber terminals. Front. Cell. Neurosci. 8, 28 (2014).
Caroni, P., Donato, F. & Muller, D. Structural plasticity upon learning: regulation and functions. Nat. Rev. Neurosci. 13, 478–490 (2012).
Galván, E. J., Cosgrove, K. E. & Barrionuevo, G. Multiple forms of long-term synaptic plasticity at hippocampal mossy fiber synapses on interneurons. Neuropharmacology 60, 740–747 (2011).
McBain, C. J. Differential mechanisms of transmission and plasticity at mossy fiber synapses. Prog. Brain Res. 169, 225–240 (2008).
Gabernet, L., Jadhav, S. P., Feldman, D. E., Carandini, M. & Scanziani, M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron 48, 315–327 (2005).
Buzsáki, G. & Wang, X.-J. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 35, 203–225 (2012).
Isaacson, J. S. & Scanziani, M. How inhibition shapes cortical activity. Neuron 72, 231–243 (2011).
Cherubini, E. & Miles, R. The CA3 region of the hippocampus: how is it? What is it for? How does it do it? Front. Cell. Neurosci. 9, 19 (2015).
Münster-Wandowski, A., Gómez-Lira, G. & Gutiérrez, R. Mixed neurotransmission in the hippocampal mossy fibers. Front. Cell. Neurosci. 7, 210 (2013).
Bischofberger, J., Engel, D., Frotscher, M. & Jonas, P. Timing and efficacy of transmitter release at mossy fiber synapses in the hippocampal network. Pflugers Arch. 453, 361–372 (2006).
Chicurel, M. E. & Harris, K. M. Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. J. Comp. Neurol. 325, 169–182 (1992).
Rollenhagen, A. et al. Structural determinants of transmission at large hippocampal mossy fiber synapses. J. Neurosci. 27, 10434–10444 (2007).
Wilke, S. A. et al. Deconstructing complexity: serial block-face electron microscopic analysis of the hippocampal mossy fiber synapse. J. Neurosci. 33, 507–522 (2013).
Henze, D. A., Wittner, L. & Buzsáki, G. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat. Neurosci. 5, 790–795 (2002). This study demonstrates by single-cell stimulation in vivo that mossy fibre–CA3 synapses act as conditional detonators that are highly dependent on the pattern of presynaptic dentate gyrus granule cell firing.
Lanore, F. et al. Deficits in morphofunctional maturation of hippocampal mossy fiber synapses in a mouse model of intellectual disability. J. Neurosci. 32, 17882–17893 (2012).
Vyleta, N. P. & Jonas, P. Loose coupling between Ca2+ channels and release sensors at a plastic hippocampal synapse. Science 343, 665–670 (2014).
Sachidhanandam, S., Blanchet, C., Jeantet, Y., Cho, Y. H. & Mulle, C. Kainate receptors act as conditional amplifiers of spike transmission at hippocampal mossy fiber synapses. J. Neurosci. 29, 5000–5008 (2009).
Hallermann, S., Pawlu, C., Jonas, P. & Heckmann, M. A large pool of releasable vesicles in a cortical glutamatergic synapse. Proc. Natl Acad. Sci. USA 100, 8975–8980 (2003).
Chamberland, S., Evstratova, A. & Toth, K. Interplay between synchronization of multivesicular release and recruitment of additional release sites support short-term facilitation at hippocampal mossy fiber to CA3 pyramidal cells synapses. J. Neurosci. 34, 11032–11047 (2014).
Klausnitzer, J. & Manahan-Vaughan, D. Frequency facilitation at mossy fiber-CA3 synapses of freely behaving rats is regulated by adenosine A1 receptors. J. Neurosci. 28, 4836–4840 (2008).
Schmitz, D., Mellor, J., Frerking, M. & Nicoll, R. A. Presynaptic kainate receptors at hippocampal mossy fiber synapses. Proc. Natl Acad. Sci. USA 98, 11003–11008 (2001).
Contractor, A., Swanson, G. & Heinemann, S. F. Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus. Neuron 29, 209–216 (2001).
Pinheiro, P. S. et al. GluR7 is an essential subunit of presynaptic kainate autoreceptors at hippocampal mossy fiber synapses. Proc. Natl Acad. Sci. USA 104, 12181–12186 (2007).
Lauri, S. E. et al. A role for Ca2+ stores in kainate receptor-dependent synaptic facilitation and LTP at mossy fiber synapses in the hippocampus. Neuron 39, 327–341 (2003).
Scott, R., Lalic, T., Kullmann, D. M., Capogna, M. & Rusakov, D. A. Target-cell specificity of kainate autoreceptor and Ca2+-store-dependent short-term plasticity at hippocampal mossy fiber synapses. J. Neurosci. 28, 13139–13149 (2008).
Jackman, S. L., Turecek, J., Belinsky, J. E. & Regehr, W. G. The calcium sensor synaptotagmin 7 is required forsynaptic facilitation. Nature 529, 88–91 (2016). This study identifies the Ca2+ sensor synaptotagmin 7 as a major determinant of synaptic facilitation in several CNS synapses, explaining the extensive short-term facilitation at mossy fibre–CA3 PC synapses.
Geiger, J. R. & Jonas, P. Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron 28, 927–939 (2000).
Carta, M. et al. Membrane lipids tune synaptic transmission by direct modulation of presynaptic potassium channels. Neuron 81, 787–799 (2014). This study identifies a novel form of synaptic plasticity at mossy fibre–CA3 PC synapses, which is based on retrograde signalling through the activity-dependent release of arachidonic acid and the subsequent inactivation of presynaptic K+ channels.
Ruiz, A., Campanac, E., Scott, R. S., Rusakov, D. A. & Kullmann, D. M. Presynaptic GABAA receptors enhance transmission and LTP induction at hippocampal mossy fiber synapses. Nat. Neurosci. 13, 431–438 (2010).
Alle, H. & Geiger, J. R. P. Combined analog and action potential coding in hippocampal mossy fibers. Science 311, 1290–1293 (2006).
Uchida, T., Fukuda, S. & Kamiya, H. Heterosynaptic enhancement of the excitability of hippocampal mossy fibers by long-range spill-over of glutamate. Hippocampus 22, 222–229 (2010).
Hagena, H. & Manahan-Vaughan, D. Frequency facilitation at mossy fiber-CA3 synapses of freely behaving rats contributes to the induction of persistent LTD via an adenosine-A1 receptor-regulated mechanism. Cereb. Cortex 20, 1121–1130 (2010).
Frausto, S. F., Ito, K., Marszalec, W. & Swanson, G. T. A novel form of low-frequency hippocampal mossy fiber plasticity induced by bimodal mGlu1 receptor signaling. J. Neurosci. 31, 16897–16906 (2011).
Pernía-Andrade, A. J. & Jonas, P. Theta-gamma-modulated synaptic currents in hippocampal granule cells in vivo define a mechanism for network oscillations. Neuron 81, 140–152 (2014).
Fernandes, H. B. et al. Epac2 mediates cAMP-dependent potentiation of neurotransmission in the hippocampus. J. Neurosci. 35, 6544–6553 (2015).
Contractor, A. et al. Trans-synaptic Eph receptor-ephrin signaling in hippocampal mossy fiber LTP. Science 296, 1864–1869 (2002).
Armstrong, J. N. B-Ephrin reverse signaling is required for NMDA-independent long-term potentiation of mossy fibers in the hippocampus. J. Neurosci. 26, 3474–3481 (2006).
Hagena, H. & Manahan-Vaughan, D. mGlu5 acts as a switch for opposing forms of synaptic plasticity at mossy fiber-CA3 and commissural associational-CA3 synapses. J. Neurosci. 35, 4999–5006 (2015).
Hagena, H. & Manahan-Vaughan, D. Learning-facilitated long-term depression and long-term potentiation at mossy fiber-CA3 synapses requires activation of β-adrenergic receptors. Front. Integr. Neurosci. 6, 23 (2012).
Danzer, S. C. & McNamara, J. O. Localization of brain-derived neurotrophic factor to distinct terminals of mossy fiber axons implies regulation of both excitation and feedforward inhibition of CA3 pyramidal cells. J. Neurosci. 24, 11346–11355 (2004).
Li, Y., Calfa, G., Inoue, T., Amaral, M. D. & Pozzo-Miller, L. Activity-dependent release of endogenous BDNF from mossy fibers evokes a TRPC3 current and Ca2+ elevations in CA3 pyramidal neurons. J. Neurophysiol. 103, 2846–2856 (2010).
Huang, Y. Z., Pan, E., Xiong, Z.-Q. & McNamara, J. O. Zinc-mediated transactivation of TrkB potentiates the hippocampal mossy fiber-CA3 pyramid synapse. Neuron 57, 546–558 (2008).
Schjetnan, A. G.-P. & Escobar, M. L. In vivo BDNF modulation of hippocampal mossy fiber plasticity induced by high frequency stimulation. Hippocampus 22, 1–8 (2010).
Mistry, R., Dennis, S., Frerking, M. & Mellor, J. R. Dentate gyrus granule cell firing patterns can induce mossy fiber long-term potentiation in vitro. Hippocampus 21, 1157–1168 (2010).
Kwon, H.-B. & Castillo, P. E. Long-term potentiation selectively expressed by NMDA receptors at hippocampal mossy fiber synapses. Neuron 57, 108–120 (2008).
Rebola, N., Lujan, R., Cunha, R. A. & Mulle, C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron 57, 121–134 (2008). References 50 and 51 simultaneously demonstrate that NMDA-EPSCs at mossy fibre–CA3 synapses can undergo LTP.
Rebola, N., Carta, M., Lanore, F., Blanchet, C. & Mulle, C. NMDA receptor-dependent metaplasticity at hippocampal mossy fiber synapses. Nat. Neurosci. 14, 691–693 (2011).
Hunt, D. L., Puente, N., Grandes, P. & Castillo, P. E. Bidirectional NMDA receptor plasticity controls CA3 output and heterosynaptic metaplasticity. Nat. Neurosci. 16, 1049–1059 (2013). References 52 and 53 show that LTP of NMDA-EPSCs at mossy fibre–CA3 synapses has important consequences for metaplasticity and cellular computation.
Astori, S., Pawlak, V. & Köhr, G. Spike-timing-dependent plasticity in hippocampal CA3 neurons. J. Physiol. (Lond.) 588, 4475–4488 (2010).
Vergnano, A. M. et al. Zinc dynamics and action at excitatory synapses. Neuron 82, 1101–1114 (2014).
Carta, M., Fievre, S., Gorlewicz, A. & Mulle, C. Kainate receptors in the hippocampus. Eur. J. Neurosci. 39, 1835–1844 (2014).
Pinheiro, P. S. et al. Selective block of postsynaptic kainate receptors reveals their function at hippocampal mossy fiber synapses. Cereb. Cortex 23, 323–331 (2013).
Selak, S. et al. A role for SNAP25 in internalization of kainate receptors and synaptic plasticity. Neuron 63, 357–371 (2009).
Carta, M. et al. CaMKII-dependent phosphorylation of GluK5 mediates plasticity of kainate receptors. EMBO J. 32, 496–510 (2013).
Chamberlain, S. E. L. et al. SUMOylation and phosphorylation of GluK2 regulate kainate receptor trafficking and synaptic plasticity. Nat. Neurosci. 15, 845–852 (2012).
Chamberlain, S. E. L., Sadowski, J. H. L. P., Teles-Grilo Ruivo, L. M., Atherton, L. A. & Mellor, J. R. Long-term depression of synaptic kainate receptors reduces excitability by relieving inhibition of the slow afterhyperpolarization. J. Neurosci. 33, 9536–9545 (2013).
Zalutsky, R. A. & Nicoll, R. A. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science 248, 1619–1624 (1990).
Tsukamoto, M. et al. Mossy fibre synaptic NMDA receptors trigger non-Hebbian long-term potentiation at entorhino-CA3 synapses in the rat. J. Physiol. (Lond.) 546, 665–675 (2003).
Debanne, D., Gähwiler, B. H. & Thompson, S. M. Long-term synaptic plasticity between pairs of individual CA3 pyramidal cells in rat hippocampal slice cultures. J. Physiol. (Lond.) 507, 237–247 (1998).
Kobayashi, K. & Poo, M.-M. Spike train timing-dependent associative modification of hippocampal CA3 recurrent synapses by mossy fibers. Neuron 41, 445–454 (2004).
Mishra, R. K., Kim, S., Guzman, S. J. & Jonas, P. Symmetric spike timing-dependent plasticity at CA3–CA3 synapses optimizes storage and recall in autoassociative networks. Nat. Commun. 7, 11552 (2016). This study demonstrates that STDPat CA3–CA3 synapses occurs independently of temporal order and proposes that the symmetric STDP rule enables reliable storage of information in the autoassociative network.
Brown, J. T. & Randall, A. D. Activity-dependent depression of the spike after-depolarization generates long-lasting intrinsic plasticity in hippocampal CA3 pyramidal neurons. J. Physiol. (Lond.) 587, 1265–1281 (2009).
Do, V. H., Martinez, C. O., Martinez, J. L. & Derrick, B. E. Long-term potentiation in direct perforant path projections to the hippocampal CA3 region in vivo. J. Neurophysiol. 87, 669–678 (2002).
McMahon, D. B. T. & Barrionuevo, G. Short- and long-term plasticity of the perforant path synapse in hippocampal area CA3. J. Neurophysiol. 88, 528–533 (2002).
Martinez, C. O., Do, V. H. & Derrick, B. E. Neurobiology of learning and memory. Neurobiol. Learn. Mem. 96, 207–217 (2011).
Major, G., Larkum, M. E. & Schiller, J. Active properties of neocortical pyramidal neuron dendrites. Annu. Rev. Neurosci. 36, 1–24 (2013).
Kleindienst, T., Winnubst, J., Roth-Alpermann, C., Bonhoeffer, T. & Lohmann, C. Activity-dependent clustering of functional synaptic inputs on developing hippocampal dendrites. Neuron 72, 1012–1024 (2011).
Takahashi, N. et al. Locally synchronized synaptic inputs. Science 335, 353–356 (2012).
Perez-Rosello, T. et al. Passive and active shaping of unitary responses from associational/commissural and perforant path synapses in hippocampal CA3 pyramidal cells. J. Comput. Neurosci. 31, 159–182 (2011).
Makara, J. K. & Magee, J. C. Variable dendritic integrationin hippocampal CA3 pyramidal neurons. Neuron 80, 1438–1450 (2013).
Kim, S., Guzman, S. J., Hu, H. & Jonas, P. Active dendrites support efficient initiation of dendritic spikes in hippocampal CA3 pyramidal neurons. Nat. Neurosci. 15, 600–606 (2012).
Hyun, J. H., Eom, K., Lee, K.-H., Ho, W.-K. & Lee, S.-H. Activity-dependent downregulation of D-type K+ channel subunit Kv1.2 in rat hippocampal CA3 pyramidal neurons. J. Physiol. (Lond.) 591, 5525–5540 (2013).
Takahashi, H. & Magee, J. C. Pathway interactions and synaptic plasticity in the dendritic tuft regions of CA1 pyramidal neurons. Neuron 62, 102–111 (2009).
Brandalise, F. & Gerber, U. Mossy fiber-evoked subthreshold responses induce timing-dependent plasticity at hippocampal CA3 recurrent synapses. Proc. Natl Acad. Sci. USA 111, 4303–4308 (2014).
Kaifosh, P. & Losonczy, A. Mnemonic functions for nonlinear dendritic integration in hippocampal pyramidal circuits. Neuron 90, 622–634 (2016).
Hyun, J. H. et al. Kv1.2 mediates heterosynaptic modulation of direct cortical synaptic inputs in CA3 pyramidal cells. J. Physiol. (Lond.) 593, 3617–3643 (2015). This study shows that activity-dependent downregulation of Kv1.2 in distal dendrites of CA3 PCs mediates heterosynaptic plasticity at PP synapses induced by mossy fibres.
Szabó, G. G., Papp, O. I., Máté, Z., Szabó, G. & Hájos, N. Anatomically heterogeneous populations of CB 1cannabinoid receptor-expressing interneurons in the CA3 region of the hippocampus show homogeneous input-output characteristics. Hippocampus 24, 1506–1523 (2014).
Hajos, N. et al. Input-output features of anatomically identified CA3 neurons during hippocampal sharp wave/ripple oscillation in vitro. J. Neurosci. 33, 11677–11691 (2013).
Losonczy, A., Biró, A. A. & Nusser, Z. Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc. Natl Acad. Sci. USA 101, 1362–1367 (2004).
Schlingloff, D., Kali, S., Freund, T. F., Hajos, N. & Gulyas, A. I. Mechanisms of sharp wave initiation and ripple generation. J. Neurosci. 34, 11385–11398 (2014).
Freund, T. F. & Katona, I. Perisomatic inhibition. Neuron 56, 33–42 (2007).
Hájos, N. et al. Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J. Neurosci. 24, 9127–9137 (2004).
Torborg, C. L., Nakashiba, T., Tonegawa, S. & McBain, C. J. Control of CA3 output by feedforward inhibition despite developmental changes in the excitation-inhibition balance. J. Neurosci. 30, 15628–15637 (2010).
Szabadics, J. & Soltesz, I. Functional specificity of mossy fiber innervation of GABAergic cells in the hippocampus. J. Neurosci. 29, 4239–4251 (2009).
Lawrence, J. J. & McBain, C. J. Interneuron diversity series: containing the detonation — feedforward inhibition in the CA3 hippocampus. Trends Neurosci. 26, 631–640 (2003).
Zucca, S. et al. Control of spike transfer at hippocampal mossy fiber synapses in vivo by GABAA and GABAB receptor mediated inhibition. J. Neurosci. 37, 587–598 (2017).
Mori, M., Abegg, M. H., Gähwiler, B. H. & Gerber, U. A frequency-dependent switch from inhibition to excitation in a hippocampal unitary circuit. Nature 431, 453–456 (2004).
Toth, K., Suares, G., Lawrence, J. J., Philips-Tansey, E. & McBain, C. J. Differential mechanisms of transmission at three types of mossy fiber synapse. J. Neurosci. 20, 8279–8289 (2000).
Lei, S. & McBain, C. J. Two loci of expression for long-term depression at hippocampal mossy fiber-interneuron synapses. J. Neurosci. 24, 2112–2121 (2004).
Pelkey, K. A., Lavezzari, G., Racca, C., Roche, K. W. & McBain, C. J. mGluR7 is a metaplastic switch controlling bidirectional plasticity of feedforward inhibition. Neuron 46, 89–102 (2005).
Galván, E. J., Calixto, E. & Barrionuevo, G. Bidirectional Hebbian plasticity at hippocampal mossy fiber synapses on CA3 interneurons. J. Neurosci. 28, 14042–14055 (2008).
Csicsvari, J., Jamieson, B., Wise, K. D. & Buzsáki, G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron 37, 311–322 (2003).
Bazelot, M., Dinocourt, C., Cohen, I. & Miles, R. Unitary inhibitory field potentials in the CA3 region of rat hippocampus. J. Physiol. (Lond.) 588, 2077–2090 (2010).
Beyeler, A. et al. Recruitment of perisomatic inhibition during spontaneous hippocampal activity in vitro. PLoS ONE 8, e66509 (2013).
Dugladze, T., Schmitz, D., Whittington, M. A., Vida, I. & Gloveli, T. Segregation of axonal and somatic activity during fast network oscillations. Science 336, 1458–1461 (2012).
Jadhav, S. P., Kemere, C., German, P. W. & Frank, L. M. Awake hippocampal sharp-wave ripples support spatial memory. Science 336, 1454–1458 (2012).
Csicsvari, J., Hirase, H., Mamiya, A. & Buzsáki, G. Ensemble patterns of hippocampal CA3-CA1 neurons during sharp wave-associated population events. Neuron 28, 585–594 (2000).
Bazelot, M., Teleñczuk, M. T. & Miles, R. Single CA3 pyramidal cells trigger sharp waves in vitroby exciting interneurones. J. Physiol. (Lond.) 594, 2565–2577 (2016).
Kohus, Z. et al. Properties and dynamics of inhibitory synaptic communication within the CA3 microcircuits of pyramidal cells and interneurons expressing parvalbumin or cholecystokinin. J. Physiol. (Lond.) 594, 3745–3774 (2016).
Viney, T. J. et al. Network state-dependent inhibition of identified hippocampal CA3 axo-axonic cells in vivo. Nat. Neurosci. 16, 1802–1811 (2013).
Kesner, R. P. A process analysis of the CA3 subregion of the hippocampus. Front. Cell. Neurosci. 7, 78 (2013).
Rennó-Costa, C., Lisman, J. E. & Verschure, P. F. M. J. A signature of attractor dynamics in the CA3 region of the hippocampus. PLoS Comput. Biol. 10, e1003641 (2014).
Knierim, J. J. & Zhang, K. Attractor dynamics of spatially correlated neural activity in the limbic system. Annu. Rev. Neurosci. 35, 267–285 (2012).
Neher, T., Cheng, S. & Wiskott, L. Memory storage fidelity in the hippocampal circuit: the role of subregions and input statistics. PLoS Comput. Biol. 11, e1004250 (2015).
Guzman, S. J., Schlögl, A., Frotscher, M. & Jonas, P. Synaptic mechanisms of pattern completion in the hippocampal CA3 network. Science 353, 1117–1123 (2016). Combining functional connectivity analysis and modelling, this study shows a high enrichment in specific unitary connection motifs between CA3 neurons, which is proposed to serve as efficient memory storage.
Guzowski, J. F., Knierim, J. J. & Moser, E. I. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron 44, 581–584 (2004).
Treves, A. & Rolls, E. T. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus 2, 189–199 (1992).
Leutgeb, S., Leutgeb, J. K., Treves, A., Moser, M.-B. & Moser, E. I. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science 305, 1295–1298 (2004).
Lee, I., Rao, G. & Knierim, J. J. A double dissociation between hippocampal subfields: differential time course of CA3 and CA1 place cells for processing changed environments. Neuron 42, 803–815 (2004).
Leutgeb, J. K., Leutgeb, S., Moser, M.-B. & Moser, E. I. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 315, 961–966 (2007).
Neunuebel, J. P. & Knierim, J. J. CA3 retrieves coherent representations from degraded input: direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron 81, 416–427 (2014). By simultaneous recording of CA3 and dentate gyrus cells in behaving rats, this study confirms a distinct contribution of CA3 and dentate gyrus cells to pattern completion and pattern separation, respectively.
Vazdarjanova, A. & Guzowski, J. F. Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J. Neurosci. 24, 6489–6496 (2004).
Niibori, Y. et al. Suppression of adult neurogenesis impairs population coding of similar contexts in hippocampal CA3 region. Nat. Commun. 3, 1253 (2012).
Jezek, K., Henriksen, E. J., Treves, A., Moser, E. I. & Moser, M.-B. Theta-paced flickering between place-cell maps in the hippocampus. Nature 478, 246–249 (2012).
Leutgeb, S., Leutgeb, J. K., Moser, E. I. & Moser, M.-B. Fast rate coding in hippocampal CA3 cell ensembles. Hippocampus 16, 765–774 (2006).
Kesner, R. P. Behavioral functions of the CA3 subregion of the hippocampus. Learn. Mem. 14, 771–781 (2007).
Nakashiba, T., Buhl, D. L., McHugh, T. J. & Tonegawa, S. Hippocampal CA3 output is crucial for ripple- associated reactivation and consolidation of memory. Neuron 62, 781–787 (2009).
Nakazawa, K. et al. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron 38, 305–315 (2003). This study shows that mice deficient for NMDARs in CA3 PCs are deficient in the rapid encoding of one-time experience.
Kesner, R. P. & Warthen, D. K. Implications of CA3 NMDA and opiate receptors for spatial pattern completion in rats. Hippocampus 20, 550–557 (2010).
McHugh, T. J. & Tonegawa, S. CA3 NMDA receptors are required for the rapid formation of a salient contextual representation. Hippocampus 19, 1153–1158 (2009).
Nakazawa, K. et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 297, 211–218 (2002).
Viana da Silva, S. et al. Early synaptic deficits in the APP/PS1 mouse model of Alzheimer's disease involve neuronal adenosine A2A receptors. Nat. Commun. 7, 11915 (2016).
Villarreal, D. M., Gross, A. L. & Derrick, B. E. Modulation of CA3 afferent inputs by novelty and theta rhythm. J. Neurosci. 27, 13457–13467 (2007).
Hayashi-Takagi, A. et al. Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 525, 333–338 (2015).
Nakashiba, T. et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell 149, 188–201 (2012).
Lassalle, J. Reversible inactivation of the hippocampal mossy fiber synapses in mice impairs spatial learning, but neither consolidation nor memory retrieval, in the Morris navigation task. Neurobiol. Learn. Mem. 73, 243–257 (2000).
Daumas, S., Ceccom, J., Halley, H., Frances, B. & Lassalle, J. M. Activation of metabotropic glutamate receptor type 2/3 supports the involvement of the hippocampal mossy fiber pathway on contextual fear memory consolidation. Learn. Mem. 16, 504–507 (2009).
Remaud, J. et al. Anisomycin injection in area CA3 of the hippocampus impairs both short-term and long-term memories of contextual fear. Learn. Mem. 21, 311–315 (2014).
Groves, J. O. et al. Ablating adult neurogenesis in the rat has no effect on spatial processing: evidence from a novel pharmacogenetic model. PLoS Genet. 9, e1003718 (2013).
Toni, N. et al. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat. Neurosci. 11, 901–907 (2008).
Sahay, A. et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472, 466–470 (2011).
Tronel, S. et al. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus 22, 292–298 (2010).
Clelland, C. D. et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213 (2009).
Liu, X. et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012). This study shows that optogenetic activation of dentate gyrus neurons tagged during one-trial contextual fear conditioning is sufficient to induce freezing behaviour.
Otto, C. et al. Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating polypeptide type I receptor-deficient mice. J. Neurosci. 21, 5520–5527 (2001).
Jones, B. W. et al. Targeted deletion of AKAP7 in dentate granule cells impairs spatial discrimination. eLife 5, 589 (2016).
Gruart, A., Sánchez-Campusano, R., Fernández-Guizán, A. & Delgado-García, J. M. A. Differential and timed contribution of identified hippocampal synapses to associative learning in mice. Cereb. Cortex 25, 2542–2555 (2015).
Routtenberg, A. Adult learning and remodeling of hippocampal mossy fibers: unheralded participant in circuitry for long-lasting spatial memory. Hippocampus 20, 44–45 (2010).
Holahan, M. R., Rekart, J. L., Sandoval, J. & Routtenberg, A. Spatial learning induces presynaptic structural remodeling in the hippocampal mossy fiber system of two rat strains. Hippocampus 16, 560–570 (2006).
Ruediger, S. et al. Learning-related feedforward inhibitory connectivity growth required for memory precision. Nature 473, 514–518 (2011). This study demonstrates that learning induces robust structural plasticity of synapses underlying feedforward inhibition in CA3 PCs and establishes a link between structural plasticity and the precision of memory.
Hagena, H. & Manahan-Vaughan, D. Learning-facilitated synaptic plasticity at CA3 mossy fiber and commissural-associational synapses reveals different roles in information processing. Cereb. Cortex 21, 2442–2449 (2011).
Ryan, T. J., Roy, D. S., Pignatelli, M., Arons, A. & Tonegawa, S. Memory. Engram cells retain memory under retrograde amnesia. Science 348, 1007–1013 (2015).
Hu, H., Gan, J. & Jonas, P. Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science 345, 1255263 (2014).
Donato, F., Rompani, S. B. & Caroni, P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature 504, 272–276 (2013).
Kubik, S., Miyashita, T. & Guzowski, J. F. Using immediate-early genes to map hippocampal subregional functions. Learn. Mem. 14, 758–770 (2007).
Lin, Y. et al. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature 455, 1198–1204 (2008).
Ramamoorthi, K. et al. Npas4 regulates a transcriptional program in CA3 required for contextual memory formation. Science 334, 1669–1675 (2011).
Nakashiba, T., Young, J. Z., McHugh, T. J., Buhl, D. L. & Tonegawa, S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science 319, 1260–1264 (2008).
Denny, C. A. et al. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron 83, 189–201 (2014).
Shipton, O. A. et al. Left-right dissociation of hippocampal memory processes in mice. Proc. Natl Acad. Sci. USA 111, 15238–15243 (2014).
Ramirez, S., Tonegawa, S. & Liu, X. Identification and optogenetic manipulation of memory engrams in the hippocampus. Front. Behav. Neurosci. 7, 226 (2013).
Kitamura, T. et al. Entorhinal cortical ocean cells encode specific contexts and drive context-specific fear memory. Neuron 87, 1317–1331 (2015).
Mori, M., Gähwiler, B. H. & Gerber, U. Recruitment of an inhibitory hippocampal network after bursting in a single granule cell. Proc. Natl Acad. Sci. USA 104, 7640–7645 (2007).
Acknowledgements
The authors are grateful to A. Treves and E. Einarsson for initial discussions on the manuscript. The work was supported by the Centre National de la Recherche Scientifique and by the Agence Nationale de la Recherche (grant Hippencode).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Neural ensembles
-
Populations of neurons that are involved in particular and/or specific neural computations.
- Associative memories
-
Memories that enable an individual to learn and remember the relationship between unrelated items.
- Attractor network
-
A neural network that has one or more stable 'states' (that is, patterns of firing across neurons). The stable states are determined by the strengths of the recurrent connections between the neurons in the network. Depending on the initial conditions, the network will end up in one of the stable states. This can allow pattern completion to occur.
- Short-term plasticity
-
A phenomenon in which synaptic efficacy changes over time in a way that reflects the history of presynaptic activity. The duration of such plasticity varies from a few milliseconds to tens of minutes.
- Long-term depression
-
Long-lasting weakening of synaptic strength between neurons, often resulting from asynchronous presynaptic and postsynaptic activity.
- Long-term potentiation
-
A persistent enhancement of excitatory synaptic transmission lasting from hours to days, triggered by strong, typically high-frequency, afferent stimulation of the synapse. It is widely studied as a putative physiological basis of long-term memory.
- Structural plasticity
-
Morphological changes that are observed in synapses, dendrites or axons following a particular stimulation protocol or a memory paradigm. The morphological changes may have functional consequences.
- En passant boutons
-
From the French for 'passing' and 'button', swellings on an axon that make non-terminal synaptic contacts on another neuron.
- Release probability
-
The probability that a single presynaptic spike will result in the release of a vesicle of neurotransmitter into the synaptic cleft. Release probability is determined by multiple presynaptic factors.
- Metaplastic switch
-
A plastic change that modifies the ability of a synapse to undergo subsequent synaptic plasticity.
- Spike-timing dependent plasticity
-
(STDP). A form of plasticity that results from functional changes in neurons and/or synapses and that depends on the precise timing of action potentials in connected neurons.
- Hebbian synaptic plasticity
-
A form of neuronal plasticity in which a change in a property (often synaptic strength) results from the simultaneous activation (sometimes repetitively) of presynaptic and postsynaptic cells.
- Pattern completion
-
The process through which a memory can be recalled by the presentation of only a subset of the cues that were available during the learning episode. There is evidence that the CA3 subregion of the hippocampus is necessary for animals to achieve pattern completion.
- Pattern separation
-
The process through which small differences in patterns of input activity are amplified as they propagate through a network to create distinct representations.
- Remapping
-
A reorganization in the pattern of place fields corresponding to particular place cells so that they bear no detectable resemblance to the pattern in the original environment. Rate remapping refers to an alteration in the firing rates of place cells that retain place fields in the same locations as before.
Rights and permissions
About this article
Cite this article
Rebola, N., Carta, M. & Mulle, C. Operation and plasticity of hippocampal CA3 circuits: implications for memory encoding. Nat Rev Neurosci 18, 208–220 (2017). https://doi.org/10.1038/nrn.2017.10
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn.2017.10
This article is cited by
-
Subfield-specific interneuron circuits govern the hippocampal response to novelty in male mice
Nature Communications (2024)
-
Dentate gyrus is needed for memory retrieval
Molecular Psychiatry (2024)
-
Phase information is conserved in sparse, synchronous population-rate-codes via phase-to-rate recoding
Nature Communications (2023)
-
Inhibitory control of sharp-wave ripple duration during learning in hippocampal recurrent networks
Nature Neuroscience (2023)
-
CA3 hippocampal synaptic plasticity supports ripple physiology during memory consolidation
Nature Communications (2023)