Key Points
-
The energy supply to the brain limits the timescale of the brain's information processing to the millisecond range.
-
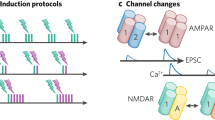
The energy supply therefore imposes a low affinity on AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptors, in order that their response is terminated in milliseconds when glutamate is removed from the synaptic cleft.
-
For diffusion to remove glutamate from the synaptic cleft in milliseconds, the diameter of synaptic boutons cannot be larger than ∼1 μm; therefore, the energy supply to the brain limits bouton size.
-
When glutamate is released at many nearby synapses, hindering clearance from the synaptic cleft by diffusion, in order for glutamate to be removed in milliseconds the initial rate of glutamate removal by transporters has evolved to occur in milliseconds; therefore, the energy supply to the brain sets the rate of this initial transporter step.
-
The high affinity of NMDA (N-methyl-D-aspartate) receptors is determined by their role as coincidence detectors on a timescale of tens of milliseconds, which requires NMDA receptor activation to last much longer than the AMPA receptor activation produced by a brief glutamate transient.
-
The ionic stoichiometry of glutamate transporters, with three Na+ ions being co-transported with each glutamate anion, is set by the need to reduce the extracellular glutamate concentration below the sub-micromolar value that will activate high-affinity NMDA receptors. Therefore, the stoichiometry is directly determined by the timescale on which NMDA receptors mediate coincidence detection.
Abstract
Why is the characteristic timescale of neural information processing in the millisecond range, corresponding to a 'clock speed' of about 1 kHz, whereas the clock speed of modern computers is about 3 GHz? Here we investigate how the brain's energy supply limits the maximum rate at which the brain can compute, and how the molecular components of excitatory synapses have evolved properties that are matched to the information processing they perform.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sarpeshkar, R. Analog versus digital: extrapolating from electronics to neurobiology. Neural Comput. 10, 1601–1638 (1998). Suggests that the division of neural processing into 'analogue' (dendrites) and 'digital' (axons) modes reflects evolution's minimization of brain energy use.
Aiello, L. C. & Wheeler, P. The expensive-tissue hypothesis: the brain and the digestive system in human evolution. Curr. Anthropol. 36, 199–221 (1995).
Attwell, D. & Laughlin, S. B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 21, 1133–1145 (2001).
Mitchison, G. Axonal trees and cortical architecture. Trends Neurosci. 15, 122–126 (1992).
Koulakov, A. A. & Chklovskii, D. B. Orientation preference patterns in mammalian visual cortex: a wire length minimization approach. Neuron 29, 519–527 (2001).
Levy, W. B. & Baxter, R. A. Energy efficient neural codes. Neural Comput. 8, 531–543 (1996).
Baddeley, R. et al. Responses of neurons in primary and inferior temporal visual cortices to natural scenes. Proc. R. Soc. Lond. B 264, 1775–1783 (1997).
Laughlin, S. B. & Sejnowski, T. J. Communication in neuronal networks. Science 301, 1870–1874 (2003). A review that explains a wide range of CNS design features.
Koch, C., Rapp, M. & Segev, I. A brief history of time (constants). Cereb. Cortex 6, 93–101 (1996).
Weckstrom, M. & Laughlin, S. B. Visual ecology and voltage-gated ion channels in insect photoreceptors. Trends Neurosci. 18, 17–21 (1995). A review that analyses how the membrane conductances of insect neurons have kinetics matched to the information processing they perform.
Lennie, P. The cost of cortical computation. Curr. Biol. 13, 493–497 (2003). Analyses the energy expended on different cellular mechanisms underlying signal processing in the primate cortex.
Patneau, D. K. & Mayer, M. L. Kinetic analysis of interactions between kainate and AMPA: evidence for activation of a single receptor in mouse hippocampal neurons. Neuron 6, 785–798 (1991). Presents a simple kinetic scheme that captures many important features of AMPA receptor behaviour.
Partin, K. M., Fleck, M. W. & Mayer, M. L. AMPA receptor flip/flop mutants affecting deactivation, desensitization, and modulation by cyclothiazide, aniracetam, and thiocyanate. J. Neurosci. 16, 6634–6647 (1996).
Krampfl, K. et al. Control of kinetic properties of GluR2 flop AMPA-type channels: impact of R/G nuclear editing. Eur. J. Neurosci. 15, 51–62 (2002).
Bergles, D. E., Diamond, J. S. & Jahr, C. E. Clearance of glutamate inside the synapse and beyond. Curr. Opin. Neurobiol. 9, 293–298 (1999).
Shepherd, G. M. G. & Harris, K. M. Three dimensional structure and composition of CA3–CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J. Neurosci. 18, 8300–8310 (1998).
Rossi, D. J., Alford, S., Mugnaini, E. & Slater, N. T. Properties of transmission at a giant glutamatergic synapse in cerebellum: the mossy fibre–unipolar brush cell synapse. J. Neurophysiol. 74, 24–42 (1995).
Kinney, G. A., Overstreet, L. S. & Slater, N. T. Prolonged physiological entrapment of glutamate in the synaptic cleft of cerebellar unipolar brush cells. J. Neurophysiol. 78, 1320–1333 (1997).
Wadiche, J. I., Arriza, J. L., Amara, S. G. & Kavanaugh, M. P. Kinetics of a human glutamate transporter. Neuron 14, 1019–1027 (1995).
Otis, T. S. & Kavanaugh, M. P. Isolation of current components and partial reaction cycles in the glial glutamate transporter EAAT2. J. Neurosci. 20, 2749–2757 (2000). Demonstrates rapid removal of glutamate by the most important glutamate transporter in the brain.
Grewer, C., Watzke, N., Wiessner, M. & Rauen, T. Glutamate translocation of the neuronal glutamate transporter EAAC1 ocurs within milliseconds. Proc. Natl Acad. Sci. USA 97, 9706–9711 (2000).
Auger, C. & Attwell, D. Fast removal of synaptic glutamate by postsynaptic transporters. Neuron 28, 547–558 (2000).
Lehre, K. P. & Danbolt, N. C. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J. Neurosci. 18, 8751–8757 (1998).
Dehnes, Y. et al. The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: a glutamate-gated chloride channel concentrated near the synapse in parts of the dendritic membrane facing astroglia. J. Neurosci. 18, 3606–3619 (1998).
Lester, R. A. & Jahr, C. E. NMDA channel behavior depends on agonist affinity. J. Neurosci. 12, 635–643 (1992). Presents a simple kinetic scheme that captures many important features of NMDA receptor behaviour.
Clements, J. D. & Westbrook, G. L. Activation kinetics reveal the number of glutamate and glycine binding sites on the N-methyl-D-aspartate receptor. Neuron 7, 605–613 (1991).
Popescu, G. & Auerbach, A. Modal gating of NMDA receptors and the shape of their synaptic response. Nature Neurosci. 6, 476–483 (2003).
Bliss, T. V. & Collingridge, G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39 (1993).
Markram, H., Lubke, J., Frotscher, M. & Sakmann, B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275, 178–179 (1997).
Zerangue, N. & Kavanaugh, M. P. Flux coupling in a neuronal glutamate transporter. Nature 383, 634–637 (1996).
Levy, L. M., Warr, O. & Attwell, D. Stoichiometry of the glial glutamate transporter GLT-1 expressed inducibly in a Chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. J. Neurosci. 18, 9620–9628 (1998).
Conn, P. J. & Pin, J. P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 37, 205–237 (1997).
Toms, N. J., Jane, D. E., Tse, H. W. & Roberts, P. J. Characterization of metabotropic glutamate receptor-stimulated phosphoinositide hydrolysis in rat cultured cerebellar granule cells. Br. J. Pharmacol. 116, 2824–2827 (1995).
Makoff, A., Lelchuk, R., Oxer, M., Harrington, K. & Emson, P. Molecular characterization and localization of human metabotropic glutamate receptor type 4. Brain Res. Mol. Brain Res. 37, 239–248 (1996).
Lerma, J., Paternain, A. V., Rodriguez-Moreno, A. & Lopez-Garcia, J. C. Molecular physiology of kainate receptors. 81, 971–998 (2001).
Colquhoun, D., Jonas, P. & Sakmann, B. Action of brief pulses of glutamate on AMPA/kainate receptors in patches from different neurones of rat hippocampal slices. J. Physiol. (Lond.) 458, 261–287 (1992).
Zorumski, C. F., Mennerick, S. & Que, J. Modulation of excitatory synaptic transmission by low concentrations of glutamate in cultured rat hippocampal neurons. J. Physiol. (Lond.) 494, 465–477 (1996).
Jones, K. A., Wilding, T. J., Huettner, J. E. & Costa, A. M. Desensitization of kainate receptors by kainate, glutamate and diastereomers of 4-methylglutamate. Neuropharmacology 36, 853–863 (1997).
Wilding, T. J. & Huettner, J. E. Activation and desensitization of hippocampal kainate receptors. J. Neurosci. 17, 2713–2721 (1997).
Paternain, A. V., Rodriguez-Moreno, A., Villarroel, A. & Lerma, J. Activation and desensitization properties of native and recombinant kainate receptors. Neuropharmacology 37, 1249–1259 (1998).
Choi, D. W. Ionic dependence of glutamate neurotoxicity. J. Neurosci. 7, 369–379 (1987).
Barbour, B. An evaluation of synapse independence. J. Neurosci. 21, 7969–7984 (2001).
Heckmann, M., Bufler, J., Franke, C. & Dudel, J. Kinetics of homomeric GluR6 glutamate receptor channels. Biophys. J. 71, 1743–1750 (1996).
Swanson, G. T. & Heinemann, S. F. Heterogeneity of homomeric GluR5 kainate receptor desensitization expressed in HEK293 cells. J. Physiol. (Lond.) 513, 639–646 (1998).
Castillo, P. E., Malenka, R. C. & Nicoll, R. A. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature 388, 182–186 (1997).
Vignes, M. & Collingridge, G. L. The synaptic activation of kainate receptors. Nature 388, 179–182 (1997).
Kidd, F. L. & Isaac, J. T. Developmental and activity-dependent regulation of kainate receptors at thalamocortical synapses. Nature 400, 569–573 (1999).
Kidd, F. L. & Isaac, J. T. Kinetics and activation of postsynaptic kainate receptors at thalamocortical synapses: role of glutamate clearance. J. Neurophysiol. 86, 1139–1148 (2001).
Li, P. et al. Kainate-receptor-mediated sensory synaptic transmission in mammalian spinal cord. Nature 397, 161–164 (1999).
Frerking, M. & Ohliger-Frerking, P. AMPA receptors and kainate receptors encode different features of afferent activity. J. Neurosci. 22, 7434–7443 (2002).
Batchelor, A. M. & Garthwaite, J. Frequency detection and temporally dispersed synaptic signal association through a metabotropic glutamate receptor pathway. Nature 385, 74–77 (1997).
Shen, K. Z. & Johnson, S. W. A slow excitatory postsynaptic current mediated by G-protein-coupled metabotropic glutamate receptors in rat ventral tegmental dopamine neurons. Eur. J. Neurosci. 9, 48–54 (1997).
Tempia, F., Miniaci, M. C., Anchisi, D. & Strata, P. Postsynaptic current mediated by metabotropic glutamate receptors in cerebellar Purkinje cells. J. Neurophysiol. 80, 520–528 (1998).
Kammermeier, P. J. & Ikeda, S. R. Desensitization of group I metabotropic glutamate receptors in rat sympathetic neurons. J. Neurophysiol. 87, 1669–1676 (2002).
Guatteo, E., Mercuri, N. B., Bernardi, G. & Knopfel, T. Group I metabotropic glutamate receptors mediate an inward current in rat substantia nigra dopamine neurons that is independent from calcium mobilization. J. Neurophysiol. 82, 1974–1981 (1999).
Borowsky, I. W. & Collins, R. C. Metabolic anatomy of brain: a comparison of regional capillary density, glucose metabolism, and enzyme activities. J. Comp. Neurol. 288, 401–413 (1989).
Zeki, S. Localization and globalization in consciousness vision. Annu. Rev. Neurosci. 24, 57–86 (2001).
Hestrin, S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature 357, 686–689 (1992).
Flint, A. C., Maisch, U. S., Weishaupt, J. H., Kriegstein, A. R. & Monyer, H. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J. Neurosci. 17, 2469–2476 (1997).
Barlow, H. B. What causes trichromacy? A theoretical analysis using comb-filtered spectra. Vision Res. 22, 635–643 (1982). This paper analyses how the sampling range of a set of photoreceptors dictates how many different receptor types it is worth expressing to sample the whole wavelength range: a problem analogous to deciding how many glutamate receptor subtypes should be expressed to sample synaptically released glutamate.
Collingridge, G. L., Herron, C. E. & Lester, R. A. Frequency-dependent N-methyl-D-aspartate receptor-mediated synaptic transmission in rat hippocampus. J. Physiol. (Lond.) 399, 301–312 (1988).
Dingledine, R., Hynes, M. A. & King, G. L. Involvement of N-methyl-D-aspartate receptors in epileptiform bursting in the rat hippocampal slice. J. Physiol. (Lond.) 380, 175–189 (1986).
Weibel, E. R. Symmorphosis: on Form and Function in Shaping Life (Harvard Univ. Press, USA, 2000).
Gardner, S. M., Trussell, L. O. & Oertel, D. Correlation of AMPA receptor subunit composition with synaptic input in the mammalian cochlear nuclei. J. Neurosci. 21, 7428–7437 (2001).
Walker, H. C., Lawrence, J. J. & McBain, C. J. Activation of kinetically distinct synaptic conductances on inhibitory interneurons by electrotonically overlapping afferents. Neuron 35, 161–171 (2002).
Sterling, P. & Matthews, G. Structure and function of ribbon synapses. Trends Neurosci. 28, 20–29 (2005).
Attwell, D., Barbour, B. & Szatkowski, M. Nonvesicular release of neurotransmitter. Neuron 11, 401–407 (1993).
Faisal, A. A., White, J. A. & Laughlin, S. B. Ion channel noise places limits on the miniaturization of the brain's wiring. Curr. Biol. 15, 1143–1149 (2005).
Hoge, R. D. et al. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc. Natl Acad. Sci. USA 96, 9403–9408 (1999).
Buchweitz, E., Sinha, A. K. & Weiss, H. R. Cerebral regional oxygen consumption and supply in anesthetized cat. Science 209, 499–501 (1980).
Hertz, M. M., Paulsen, O. B., Barry, D. I., Christiansen, J. S. & Svendsen, P. A. Insulin increases glucose transfer across the blood–brain barrier in man. J. Clin. Invest. 67, 597–604 (1981).
Mandeville, J. B. et al. MRI measurement of the temporal evolution of relative CMRO2 during rat forepaw stimulation. Magn. Reson. Med. 42, 944–951 (1999).
Cavaglia, M. et al. Regional variation in brain capillary density and vascular response to ischemia. Brain Res. 910, 81–93 (2001).
Harrison, R. V., Harel, N., Panesar, J. & Mount, R. J. Blood capillary distribution correlates with hemodynamic-based functional imaging in cerebral cortex. Cereb. Cortex 12, 225–233 (2002).
Acknowledgements
We are grateful to B. Barbour, M. Häusser, S. Laughlin and A. Silver for helpful discussion. Supported by the Wellcome Trust and a Wolfson-Royal Society award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Gene
FURTHER INFORMATION
Glossary
- MEMBRANE TIME CONSTANT
-
The product of the capacitance and resistance of the cell membrane, which sets the timescale over which membrane currents change the voltage. A small time constant means that the membrane potential can change rapidly.
- MEMBRANE RESISTANCE
-
The ratio of the voltage change produced across the cell membrane to the size of current injected into the cell: the resistance is set by the number and conductance of the ion channels in the cell membrane.
- MEMBRANE CAPACITANCE
-
The cell membrane separates and stores electrical charge, thereby producing an electrical capacitance, which increases in proportion to membrane area.
- VOLTAGE-UNIFORM CELL
-
A spatially compact cell with no voltage gradients along its cytoplasm, so that the voltage across the cell membrane is the same everywhere (by contrast, cells with long dendrites are often not voltage-uniform).
- CELL RESTING POTENTIAL
-
The membrane potential at which there is no net flow of current across the cell membrane.
- NERNST POTENTIAL
-
The potential at which there is no net movement of an ionic species across the cell membrane, because the free energy decrease resulting from the ion moving down its concentration gradient is balanced by the free energy increase needed to move the ionic charge through the membrane's electric field.
- DISSOCIATION CONSTANT
-
The ratio of the unbinding rate constant (koff) to the binding rate constant (kon) when transmitter binds to a receptor.
- AMPLITUDE-WEIGHTED DECAY TIME CONSTANT
-
For a multi-exponentially decaying synaptic current, this is Σ aiτi / Σ ai, where ai and τi are the amplitude and time constant of each exponential component. An exponential decay with a time constant of this value and an amplitude Σ ai has the same area (that is, charge transfer) as the multi-exponential decay from which it is derived.
- EC50
-
The concentration of agonist that evokes a half-maximal response.
- IC50
-
The concentration of antagonist that produces a half-maximal inhibition of a response.
- Q10
-
The ratio of reaction rates for a 10°C increase in temperature.
- SYMMORPHOSIS
-
According to this theory, animal design is optimized such that structure satisfies but does not exceed functional requirements.
Rights and permissions
About this article
Cite this article
Attwell, D., Gibb, A. Neuroenergetics and the kinetic design of excitatory synapses. Nat Rev Neurosci 6, 841–849 (2005). https://doi.org/10.1038/nrn1784
Issue Date:
DOI: https://doi.org/10.1038/nrn1784
This article is cited by
-
Quantitative tracking of trans-synaptic nose-to-brain transport of nanoparticles and its modulation by odor, aging, and Parkinson’s disease
Nano Research (2023)
-
The Peculiar Facets of Nitric Oxide as a Cellular Messenger: From Disease-Associated Signaling to the Regulation of Brain Bioenergetics and Neurovascular Coupling
Neurochemical Research (2021)
-
Wrapping glia regulates neuronal signaling speed and precision in the peripheral nervous system of Drosophila
Nature Communications (2020)
-
Simultaneous voltammetric detection of glucose and lactate fluctuations in rat striatum evoked by electrical stimulation of the midbrain
Analytical and Bioanalytical Chemistry (2020)
-
Modelling acute and lasting effects of tDCS on epileptic activity
Journal of Computational Neuroscience (2020)