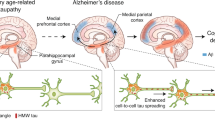
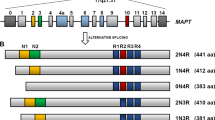
Abstract
Amyloid-β and tau are the two hallmark proteins in Alzheimer's disease. Although both amyloid-β and tau have been extensively studied individually with regard to their separate modes of toxicity, more recently new light has been shed on their possible interactions and synergistic effects in Alzheimer's disease. Here, we review novel findings that have shifted our understanding of the role of tau in the pathogenesis of Alzheimer's disease towards being a crucial partner of amyloid-β. As we gain a deeper understanding of the different cellular functions of tau, the focus shifts from the axon, where tau has a principal role as a microtubule-associated protein, to the dendrite, where it mediates amyloid-β toxicity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Citron, M. Strategies for disease modification in Alzheimer's disease. Nature Rev. Neurosci. 5, 677–685 (2004).
Brunden, K. R., Trojanowski, J. Q. & Lee, V. M. Advances in tau-focused drug discovery for Alzheimer's disease and related tauopathies. Nature Rev. Drug Discov. 8, 783–793 (2009).
Querfurth, H. W. & LaFerla, F. M. Alzheimer's disease. N. Engl. J. Med. 362, 329–344 (2010).
Cairns, N. J. et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 114, 5–22 (2007).
Braak, H. & Braak, E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol. Aging 16, 271–278; discussion 278–284 (1995).
Selkoe, D. J. Alzheimer's disease is a synaptic failure. Science 298, 789–791 (2002).
Bertram, L. & Tanzi, R. E. The genetic epidemiology of neurodegenerative disease. J. Clin. Invest. 115, 1449–1457 (2005).
Ballatore, C., Lee, V. M. & Trojanowski, J. Q. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nature Rev. Neurosci. 8, 663–672 (2007).
Götz, J. & Ittner, L. M. Animal models of Alzheimer's disease and frontotemporal dementia. Nature Rev. Neurosci. 9, 532–544 (2008).
Frost, B. & Diamond, M. I. Prion-like mechanisms in neurodegenerative diseases. Nature Rev. Neurosci. 11, 155–159 (2010).
Hardy, J. & Selkoe, D. J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002).
Sisodia, S. S. & St George-Hyslop, P. H. γ-Secretase, notch, Aβ and Alzheimer's disease: where do the presenilins fit in? Nature Rev. Neurosci. 3, 281–290 (2002).
LaFerla, F. M., Green, K. N. & Oddo, S. Intracellular amyloid-β in Alzheimer's disease. Nature Rev. Neurosci. 8, 499–509 (2007).
Haass, C. & Selkoe, D. J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid β-peptide. Nature Rev. Mol. Cell Biol. 8, 101–112 (2007).
Shankar, G. M. et al. Amyloid-β protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nature Med. 14, 837–842 (2008).
Lauren, J., Gimbel, D. A., Nygaard, H. B., Gilbert, J. W. & Strittmatter, S. M. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature 457, 1128–1132 (2009).
Snyder, E. M. et al. Regulation of NMDA receptor trafficking by amyloid-β. Nature Neurosci. 8, 1051–1058 (2005).
Kessels, H. W., Nguyen, L. N., Nabavi, S. & Malinow, R. The prion protein as a receptor for amyloid-β. Nature 466, e3–e4 (2010).
Small, D. H. et al. The β-amyloid protein of Alzheimer's disease binds to membrane lipids but does not bind to the α7 nicotinic acetylcholine receptor. J. Neurochem. 101, 1527–1538 (2007).
Roberson, E. D. et al. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer's disease mouse model. Science 316, 750–754 (2007).
Goedert, M. & Spillantini, M. G. A century of Alzheimer's disease. Science 314, 777–781 (2006).
Hirokawa, N., Funakoshi, T., Sato-Harada, R. & Kanai, Y. Selective stabilization of tau in axons and microtubule-associated protein 2C in cell bodies and dendrites contributes to polarized localization of cytoskeletal proteins in mature neurons. J. Cell Biol. 132, 667–679 (1996).
Aronov, S., Aranda, G., Behar, L. & Ginzburg, I. Axonal tau mRNA localization coincides with tau protein in living neuronal cells and depends on axonal targeting signal. J. Neurosci. 21, 6577–6587 (2001).
Utton, M. A. et al. The slow axonal transport of the microtubule-associated protein tau and the transport rates of different isoforms and mutants in cultured neurons. J. Neurosci. 22, 6394–6400 (2002).
Konzack, S., Thies, E., Marx, A., Mandelkow, E. M. & Mandelkow, E. Swimming against the tide: mobility of the microtubule-associated protein tau in neurons. J. Neurosci. 27, 9916–9927 (2007).
Ittner, L. M. et al. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer's disease mouse models. Cell 142, 387–397 (2010).
Götz, J., Ittner, L. M. & Kins, S. Do axonal defects in tau and amyloid precursor protein transgenic animals model axonopathy in Alzheimer's disease? J. Neurochem. 98, 993–1006 (2006).
Maas, T., Eidenmuller, J. & Brandt, R. Interaction of tau with the neural membrane cortex is regulated by phosphorylation at sites that are modified in paired helical filaments. J. Biol. Chem. 275, 15733–15740 (2000).
Brion, J. P., Smith, C., Couck, A. M., Gallo, J. M. & Anderton, B. H. Developmental changes in tau phosphorylation: fetal tau is transiently phosphorylated in a manner similar to paired helical filament-tau characteristic of Alzheimer's disease. J. Neurochem. 61, 2071–2080 (1993).
Götz, J. et al. Somatodendritic localization and hyperphosphorylation of tau protein in transgenic mice expressing the longest human brain tau isoform. EMBO J. 14, 1304–1313 (1995).
Santacruz, K. et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 309, 476–481 (2005).
Ittner, L. M., Ke, Y. D. & Götz, J. Phosphorylated Tau interacts with c-Jun N-terminal kinase-interacting protein 1 (JIP1) in Alzheimer disease. J. Biol. Chem. 284, 20909–20916 (2009).
Lee, G., Newman, S. T., Gard, D. L., Band, H. & Panchamoorthy, G. Tau interacts with src-family non-receptor tyrosine kinases. J. Cell Sci. 111, 3167–3177 (1998).
Magnani, E. et al. Interaction of tau protein with the dynactin complex. EMBO J. 26, 4546–4554 (2007).
Bhaskar, K., Yen, S. H. & Lee, G. Disease-related modifications in tau affect the interaction between Fyn and Tau. J. Biol. Chem. 280, 35119–35125 (2005).
Salter, M. W. & Kalia, L. V. Src kinases: a hub for NMDA receptor regulation. Nature Rev. Neurosci. 5, 317–328 (2004).
Gu, J. & Zheng, J. Q. Microtubules in dendritic spine development and plasticity. Open Neurosci. J. 3, 128–133 (2009).
Small, D. H., Mok, S. S. & Bornstein, J. C. Alzheimer's disease and Aβ toxicity: from top to bottom. Nature Rev. Neurosci. 2, 595–598 (2001).
Götz, J. et al. Transgenic animal models of Alzheimer's disease and related disorders: histopathology, behavior and therapy. Mol. Psychiatry 9, 664–683 (2004).
Lewis, J. et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293, 1487–1491 (2001).
Terwel, D. et al. Amyloid activates GSK-3β to aggravate neuronal tauopathy in bigenic mice. Am. J. Pathol. 172, 786–798 (2008).
Götz, J., Chen, F., van Dorpe, J. & Nitsch, R. M. Formation of neurofibrillary tangles in P301L tau transgenic mice induced by Aβ42 fibrils. Science 293, 1491–1495 (2001).
Oddo, S., Billings, L., Kesslak, J. P., Cribbs, D. H. & LaFerla, F. M. Aβ immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron 43, 321–332 (2004).
Coomaraswamy, J. et al. Modeling familial Danish dementia in mice supports the concept of the amyloid hypothesis of Alzheimer's disease. Proc. Natl Acad. Sci. USA 107, 7969–7974 (2010).
Rhein, V. et al. Amyloid-β and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer's disease mice. Proc. Natl Acad. Sci. USA 106, 20057–20062 (2009).
Rapoport, M., Dawson, H. N., Binder, L. I., Vitek, M. P. & Ferreira, A. Tau is essential to β-amyloid-induced neurotoxicity. Proc. Natl Acad. Sci. USA 99, 6364–6369 (2002).
Vossel, K. A. et al. Tau reduction prevents Aβ-induced defects in axonal transport. Science 330, 198 (2010).
Harada, A. et al. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature 369, 488–491 (1994).
Dawson, H. N. et al. Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J. Cell Sci. 114, 1179–1187 2001).
Tucker, K. L., Meyer, M. & Barde, Y. A. Neurotrophins are required for nerve growth during development. Nature Neurosci. 4, 29–37 (2001).
Takashima, A. The mechanism for tau aggregation and its relation to neuronal dysfunction. Alzheimer's & Dementia 6, S144 (2010)
Dawson, H. N. et al. Loss of tau elicits axonal degeneration in a mouse model of Alzheimer's disease. Neuroscience 169, 516–531 (2010).
Saper, C. B., Wainer, B. H. & German, D. C. Axonal and transneuronal transport in the transmission of neurological disease: potential role in system degenerations, including Alzheimer's disease. Neuroscience 23, 389–398 (1987).
Stamer, K., Vogel, R., Thies, E., Mandelkow, E. & Mandelkow, E. M. Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J. Cell Biol. 156, 1051–1063 (2002).
Pappolla, M. A., Omar, R. A., Kim, K. S. & Robakis, N. K. Immunohistochemical evidence of oxidative [corrected] stress in Alzheimer's disease. Am. J. Pathol. 140, 621–628 (1992).
Williamson, R., Usardi, A., Hanger, D. P. & Anderton, B. H. Membrane-bound β-amyloid oligomers are recruited into lipid rafts by a fyn-dependent mechanism. FASEB J. 22, 1552–1559 (2008).
Ittner, L. M. et al. Parkinsonism and impaired axonal transport in a mouse model of frontotemporal dementia. Proc. Natl Acad. Sci. USA 105, 15597–16002 (2008).
Gong, C. X. & Iqbal, K. Hyperphosphorylation of microtubule-associated protein tau: a promising therapeutic target for Alzheimer disease. Curr. Med. Chem. 15, 2321–2328 (2008).
Noble, W. et al. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc. Natl Acad. Sci. USA 102, 6990–6995 (2005).
van Eersel, J. et al. Sodium selenate mitigates tau pathology, neurodegeneration, and functional deficits in Alzheimer's disease models. Proc. Natl Acad. Sci. USA 107, 13888–13893 (2010).
Klein, C. et al. Process. outgrowth of oligodendrocytes is promoted by interaction of fyn kinase with the cytoskeletal protein tau. J. Neurosci. 22, 698–707 (2002).
Oddo, S. et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron 39, 409–421 (2003).
Bolmont, T. et al. Induction of tau pathology by intracerebral infusion of amyloid-β-containing brain extract and by amyloid-β deposition in APP x Tau transgenic mice. Am. J. Pathol. 171, 2012–2020 (2007).
Palop, J. J. et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron 55, 697–711 (2007).
Grueninger, F. et al. Phosphorylation of Tau at S422 is enhanced by Aβ in TauPS2APP triple transgenic mice. Neurobiol. Dis. 37, 294–306 (2010).
Acknowledgements
This work has been supported by the University of Sydney, the National Health & Medical Research Council (NHMRC), the Australian Research Council (ARC) and the J.O. & J.R. Wicking Trust.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Ittner, L., Götz, J. Amyloid-β and tau — a toxic pas de deux in Alzheimer's disease. Nat Rev Neurosci 12, 67–72 (2011). https://doi.org/10.1038/nrn2967
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn2967
This article is cited by
-
Neuropathogenesis-on-chips for neurodegenerative diseases
Nature Communications (2024)
-
Significance of Melatonin in the Regulation of Circadian Rhythms and Disease Management
Molecular Neurobiology (2024)
-
Valosin containing protein (VCP): initiator, modifier, and potential drug target for neurodegenerative diseases
Molecular Neurodegeneration (2023)
-
Alternative splicing in neurodegenerative disease and the promise of RNA therapies
Nature Reviews Neuroscience (2023)
-
Development of an accelerated cellular model for early changes in Alzheimer’s disease
Scientific Reports (2023)