Abstract
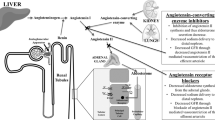
Reduction of blood pressure and proteinuria by blockade of the renin–angiotensin–aldosterone system (RAAS) has been the cornerstone of renoprotective intervention for patients with chronic kidney disease (CKD) for many years. Despite the proven efficacy of RAAS blockade, however, the reduction in proteinuria is insufficient in many patients, and does not prevent further deterioration of renal function. Short-term studies have shown that a variety of treatment intensification strategies have a beneficial effect on blood pressure and proteinuria, including RAAS blockade using either dose escalation or multiple drugs, and restriction of dietary sodium. Large clinical trials have shown that RAAS blockade with multiple drugs does not improve patients' long-term renal or cardiovascular outcome. By contrast, two post-hoc analyses of landmark trials in nephrology show beneficial renal and cardiovascular effects from avoiding excessive dietary sodium intake during single-agent RAAS blockade therapy. The effects of dietary sodium restriction on renal or cardiovascular outcome still require prospective confirmation. However, current data support the implementation of lifestyle changes to reduce dietary sodium intake in combination with single-agent RAAS blockade, rather than dual-agent RAAS blockade, as a potent and feasible strategy to mitigate the burden of renal and cardiovascular disease in patients with CKD.
Key Points
-
Despite the proven efficacy of blockade of the renin–angiontensin–aldosterone system (RAAS) as a treatment for chronic kidney disease the prognosis of these patients is poor
-
Recent data from large clinical trials demonstrate that a combination of two drugs that block the RAAS does not protect patients against renal and cardiovascular outcomes
-
Avoidance of excessive dietary sodium intake has been shown to enhance the effect of single-agent RAAS blockade against renal and cardiovascular outcomes in two post-hoc analyses of clinical trials
-
Lifestyle changes to avoid high dietary sodium intake are advised with RAAS blockade to reduce the risk of renal and cardiovascular outcomes in patients with chronic kidney disease
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care 35 (Suppl. 1), S11–S63 (2012).
Kidney Disease Outcomes Quality Initiative. Clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am. J. Kidney Dis. 43 (Suppl. 1), S1–S290 (2004).
Burgess, E. et al. Supramaximal dose of candesartan in proteinuric renal disease. J. Am. Soc. Nephrol. 20, 893–900 (2009).
Weir, M. R. et al. Antihypertensive effects of double the maximum dose of valsartan in African-American patients with type 2 diabetes mellitus and albuminuria. J. Hypertens. 28, 186–193 (2010).
Heeg, J. E., de Jong, P. E., van der Hem, G. K. & de Zeuuw, D. Efficacy and variability of the antiproteinuric effect of ACE inhibition by lisinopril. Kidney Int. 36, 272–279 (1989).
Kunz, R., Friedrich, C., Wolbers, M. & Mann, J. F. Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann. Intern. Med. 148, 30–48 (2008).
Jennings, D. L., Kalus, J. S., Coleman, C. I., Manierski, C. & Yee, J. Combination therapy with an ACE inhibitor and an angiotensin receptor blocker for diabetic nephropathy: a meta-analysis. Diabet. Med. 24, 486–493 (2007).
MacKinnon, M. et al. Combination therapy with an angiotensin receptor blocker and an ACE inhibitor in proteinuric renal disease: a systematic review of the efficacy and safety data. Am. J. Kidney Dis. 48, 8–20 (2006).
Laverman, G. D., Navis, G., Henning, R. H., de Jong, P. E. & de Zeeuw, D. Dual renin-angiotensin system blockade at optimal doses for proteinuria. Kidney Int. 62, 1020–1025 (2002).
Esnault, V. L., Ekhlas, A., Nguyen, J. M. & Moranne, O. Diuretic uptitration with half dose combined ACEI+ARB better decrease proteinuria than combined ACEI+ARB uptitration. Nephrol. Dial. Transplant. 25, 2218–2224 (2010).
Lambers-Heerspink, H. J., Perkovic V. & de Zeeuw, D. Renal and cardio-protective effects of direct renin inhibition: a systematic literature review. J. Hypertens. 27, 2321–2331 (2009).
van Paassen, P., de Zeeuw, D., Navis, G. & de Jong, P. E. Renal and systemic effects of continued treatment with renin inhibitor remikiren in hypertensive patients with normal and impaired renal function. Nephrol. Dial. Transplant. 15, 637–643 (2000).
Persson, F. et al. Renal effects of aliskiren compared with and in combination with irbesartan in patients with type 2 diabetes, hypertension, and albuminuria. Diabetes Care 32, 1873–1879 (2009).
Parving, H. H. et al. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N. Engl. J. Med. 358, 2433–2446 (2008).
Waanders, F. et al. Aldosterone, from (patho) physiology to treatment in cardiovascular and renal damage. Curr. Vasc. Pharmacol. 9, 594–605 (2011).
Blasi, E. R. et al. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 63, 1791–1800 (2003).
Nagase, M. et al. Salt-induced nephropathy in obese spontaneously hypertensive rats via paradoxical activation of the mineralocorticoid receptor: role of oxidative stress. Hypertension 50, 877–883 (2007).
Kramer, A. B., van der Meulen, E. F., Hamming, I., van Goor, H. & Navis, G. Effect of combining ACE inhibition with aldosterone blockade on proteinuria and renal damage in experimental nephrosis. Kidney Int. 71, 417–424 (2007).
Rossing, K., Schjoedt, K. J., Smidt, U. M., Boomsma, F. & Parving, H. H. Beneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: a randomized, double-masked, cross-over study. Diabetes Care 28, 2106–2112 (2005).
van den Meiracker, A. H. et al. Spironolactone in type 2 diabetic nephropathy: effects on proteinuria, blood pressure and renal function. J. Hypertens. 24, 2285–2292 (2006).
Schjoedt, K. J. et al. Beneficial impact of spironolactone on nephrotic range albuminuria in diabetic nephropathy. Kidney Int. 70, 536–542 (2006).
Epstein, M. et al. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin. J. Am. Soc. Nephrol. 1, 940–951 (2006).
Morales, E. et al. Renoprotective effect of mineralocorticoid receptor blockers in patients with proteinuric kidney disease. Nephrol. Dial. Transplant. http://dx.doi.org/10.1093/ndt/gfs429.
Navaneethan, S. D., Nigwekar, S. U., Sehgal, A. R. & Strippoli, G. F. Aldosterone antagonists for preventing the progression of chronic kidney disease: a systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 4, 542–551 (2009).
Nederlands Trial Register. trialregister.nl [online], (2012).
ONTARGET Investigators et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N. Engl. J. Med. 358, 1547–1559 (2008).
Mann, J. F. et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 372, 547–553 (2008).
Ruggenenti, P. & Remuzzi, G. Proteinuria: Is the ONTARGET renal substudy actually off target? Nat. Rev. Nephrol. 5, 436–437 (2009).
Lambers-Heerspink, H. J. & de Zeeuw, D. Dual RAS therapy not on target, but fully alive. Nephron Clin. Pract. 116, c137–c142 (2010).
Tobe, S. W. et al. Cardiovascular and renal outcomes with telmisartan, ramipril or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation 123, 1098–1107 (2011).
Parving, H. H. et al. Cardio-renal endpoints in a trial of aliskiren for type 2 diabetes. N. Engl. J. Med. http://dx.doi.org/10.1056/NEJMoa1208799.
Parving, H. H. et al. Baseline characteristics in the aliskiren trial in type 2 diabetes using cardio-renal endpoints (ALTITUDE). J. Renin Angiotensin Aldosterone Syst. 13, 387–393 (2012).
Fried, L. F. et al. Design of combination angiotensin receptor blocker and angiotensin-converting enzyme inhibitor for treatment of diabetic nephropathy (VA NEPHRON-D). Clin. J. Am. Soc. Nephrol. 4, 361–368 (2009).
Maione, A. et al. Protocol of the long-term impact of RAS inhibition on cardiorenal outcomes (LIRICO) randomized trial. J. Nephrol. 20, 646–655 (2007).
Chapman, A. B. et al. The HALT polycystic kidney disease trials: design and implementation. Clin. J. Am. Soc. Nephrol. 5, 102–109 (2010).
Gansevoort, R. T., de Zeeuw, D., Shahinfar, S., Redfield, A. & de Jong, P. E. Effects of the angiotensin II antagonist losartan in hypertensive patients with renal disease. J. Hypertens. Suppl. 12, S37–S42 (1994).
Miao, Y. et al. Increased serum potassium affects renal outcomes: a post hoc analysis of the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. Diabetologia 54, 44–50 (2011).
Slagman, M. C. et al. Erythropoietin is reduced by combination of diuretic therapy and RAAS blockade in proteinuric renal patients with preserved renal function. Nephrol. Dial. Transplant. 25, 3256–3260 (2010).
Mohanram, A., Zhang, Z., Shahinfar, S., Lyle, P. A. & Toto, R. D. The effect of losartan on hemoglobin concentration and renal outcome in diabetic nephropathy of type 2 diabetes. Kidney Int. 73, 630–636 (2008).
Miao, Y. et al. Effect of a reduction in uric acid on renal outcomes during losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension 58, 2–7 (2011).
Smink, P. A. et al. An initial reduction in serum uric acid during angiotensin receptor blocker treatment is associated with cardiovascular protection: a post-hoc analysis of the RENAAL and IDNT trials. J. Hypertens. 30, 1022–1028 (2012).
Slagman, M. C. et al. Effects of antiproteinuric intervention on elevated connective tissue growth factor (CTGF/CCN-2) plasma and urine levels in nondiabetic nephropathy. Clin. J. Am. Soc. Nephrol. 6, 1845–1850 (2011).
Onuigbo, M. A. & Onuigbo, N. T. Late-onset renal failure from angiotensin blockade (LORFFAB) in 100 CKD patients. Int. Urol. Nephrol. 40, 233–239 (2008).
Onuigbo, M. A. & Onuigbo, N. T. Worsening renal failure in older chronic kidney disease patients with renal artery stenosis concurrently on renin angiotensin aldosterone system blockade: a prospective 50-month Mayo-Health-System clinic analysis. QJM 101, 519–527 (2008).
Onuigbo, M. A. Radiographic contrast-induced nephropathy and patient mortality. Mayo Clin. Proc. 83, 1412–1413 (2008).
Richer-Giudicelli, C. et al. Haemodynamic effects of dual blockade of the renin-angiotensin system in spontaneously hypertensive rats: influence of salt. J. Hypertens. 22, 619–627 (2004).
Slagman, M. C. et al. Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: randomised controlled trial. BMJ 343, d4366 (2011).
Nallamothu, B. K., Hayward, R. A. & Bates, E. R. Beyond the randomized clinical trial: the role of effectiveness studies in evaluating cardiovascular therapies. Circulation 118, 1294–1303 (2008).
Brenner, B. M. et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 345, 861–869 (2001).
The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet 349, 1857–1863 (1997).
Navis, G., de Jong, P., Donker, A. J., van der Hem G. K. & de Zeeuw, D. Diuretic effects of angiotensin-converting enzyme inhibition: comparison of low and liberal sodium diet in hypertensive patients. J. Cardiovasc. Pharmacol. 9, 743–748 (1987).
Navis, G., de Jong, P. E., Donker, A. J., van der Hem, G. K. & de Zeeuw, D. Moderate sodium restriction in hypertensive subjects: renal effects of ACE-inhibition. Kidney Int. 31, 815–819 (1987).
MacGregor, G. A. et al. Moderate sodium restriction with angiotensin converting enzyme inhibitor in essential hypertension: a double blind study. Br. Med. J. (Clin. Res. Ed.) 294, 531–534 (1987).
Houlihan, C. A. et al. A low-sodium diet potentiates the effects of losartan in type 2 diabetes. Diabetes Care 25, 663–671 (2002).
Ekinci, E. I. et al. Effects of salt supplementation on the albuminuric response to telmisartan with or without hydrochlorothiazide therapy in hypertensive patients with type 2 diabetes are modulated by habitual dietary salt intake. Diabetes Care 32, 1398–1403 (2009).
Wapstra, F. H., Van Goor, H., Navis, G., de Jong, P. E. & de Zeeuw, D. Antiproteinuric effect predicts renal protection by angiotensin-converting enzyme inhibition in rats with established adriamycin nephrosis. Clin. Sci. (Lond.) 90, 393–401 (1996).
Vogt, L., Waanders, F., Boomsma, F., de Zeeuw, D. & Navis, G. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J. Am. Soc. Nephrol. 19, 999–1007 (2008).
Weir, M. R., Yadao, A. M., Purkayastha, D. & Charney, A. N. Effects of high- and low-sodium diets on ambulatory blood pressure in patients with hypertension receiving aliskiren. J. Cardiovasc. Pharmacol. Ther. 15, 356–363 (2010).
Buter, H., Hemmelder, M. H., Navis, G., de Jong, P. E. & de Zeeuw, D. The blunting of the antiproteinuric efficacy of ACE inhibition by high sodium intake can be restored by hydrochlorothiazide. Nephrol. Dial. Transplant. 13, 1682–1685 (1998).
Ruggenenti, P. et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 354, 359–364 (1999).
Vegter, S. et al. Sodium intake, ACE inhibition, and progression to ESRD. J. Am. Soc. Nephrol. 23, 165–173 (2011).
Lambers-Heerspink, H. J. et al. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int. 82, 330–337 (2012).
Vallon, V., Blantz, R. C. & Thomson, S. Glomerular hyperfiltration and the salt paradox in early [corrected] type 1 diabetes mellitus: a tubulo-centric view. J. Am. Soc. Nephrol. 14, 530–537 (2003).
Luik, P. T. et al. Short-term moderate sodium restriction induces relative hyperfiltration in normotensive normoalbuminuric type I diabetes mellitus. Diabetologia 45, 535–541 (2002).
Cook, N. R. et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ 334, 885–888 (2007).
Taylor, R. S., Ashton, K. E., Moxham, T., Hooper, L. & Ebrahim, S. Reduced dietary salt for the prevention of cardiovascular disease. Cochrane Database of Systematic Reviews, Issue 7. Art. No.: CD009217. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD009217/.
He, F. J. & MacGregor, G. A. Salt reduction lowers cardiovascular risk: meta-analysis of outcome trials. Lancet 378, 380–382 (2011).
Paterna, S., Gaspare, P., Fasullo, S., Sarullo, F. M. & di Pasquale, P. Normal-sodium diet compared with low-sodium diet in compensated congestive heart failure: is sodium an old enemy or a new friend? Clin. Sci. (Lond.) 114, 221–230 (2008).
Kocks, M. J., Lely, A. T., Boomsma, F., de Jong, P. E. & Navis, G. Sodium status and angiotensin-converting enzyme inhibition: effects on plasma angiotensin(1–7) in healthy man. J. Hypertens. 23, 597–602 (2005).
Kocks, M. J. et al. High dietary sodium blunts affects of angiotensin-converting enzyme inhibition on vascular angiotensin I-to-angiotensin II conversion in rats. J. Cardiovasc. Pharmacol. 42, 601–606 (2003).
Vogt, L., Kocks, M. J., Laverman, G. D. & Navis, G. Renoprotection by blockade of the renin–angiotensin–aldosterone system in diabetic and non-diabetic chronic kidney disease. Specific involvement of intra-renal angiotensin-converting enzyme activity in therapy resistance? Minerva Med. 95, 395–409 (2004).
Hamming, I., Navis, G., Kocks, M. J. & van Goor, H. ACE inhibition has adverse renal effects during dietary sodium restriction in proteinuric and healthy rats. J. Pathol. 209, 129–139 (2006).
Trongtorsak, P., Morgan, T. O. & Delbridge, L. M. Combined renin-angiotensin system blockade and dietary sodium restriction impairs cardiomyocyte contractility. J. Renin Angiotensin Aldosterone Syst. 4, 213–219 (2003).
Thomas, M. C. et al. The association between dietary sodium intake, ESRD, and all-cause mortality in patients with type 1 diabetes. Diabetes Care 34, 861–866 (2011).
O'Donnell, M. J. et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA 306, 2229–2238 (2011).
Ekinci, E. I. et al. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care 34, 703–709 (2011).
Visser, F. W., Krikken, J. A., Muntinga, J. H., Dierckx, R. A. & Navis, G. J. Rise in extracellular fluid volume during high sodium depends on BMI in healthy men. Obesity (Silver Spring) 17, 1684–1688 (2009).
Padmanabhan, S. et al. Genome-wide association study of blood pressure extremes identifies variant near UMOD associated with hypertension. PLoS Genet. 6, e1001177 (2010).
Slagman, M. C. et al. Elevated N-terminal pro-brain natriuretic peptide levels predict an enhanced anti-hypertensive and anti-proteinuric benefit of dietary sodium restriction and diuretics, but not angiotensin receptor blockade, in proteinuric renal patients. Nephrol. Dial. Transplant. 27, 983–990 (2012).
van der Kleij, F. G. et al. Angiotensin converting enzyme insertion/deletion polymorphism and short-term renal response to ACE inhibition: role of sodium status. Kidney Int. Suppl. 63, S23–S26 (1997).
Lely, T. A. et al. Response to angiotensin-converting enzyme inhibition is selectively blunted by high sodium in angiotensin-converting enzyme DD genotype: evidence for gene-environment interaction in healthy volunteers. J. Hypertens. 28, 2414–2421 (2010).
Krikken, J. A., Laverman, G. D. & Navis, G. Benefits of dietary sodium restriction in the management of chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 18, 531–538 (2009).
van den Berg, E. et al. Sodium intake and blood pressure in renal transplant recipients. Nephrol. Dial. Transplant. 27, 3352–3359 (2012).
de Zeeuw, D. et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 376, 1543–1551 (2010).
Doorenbos, C. R., van den Born, J., Navis, G. & de Borst, M. H. Possible renoprotection by vitamin D in chronic renal disease: beyond mineral metabolism. Nat. Rev. Nephrol. 5, 691–700 (2009).
Nederlands Trial Register. trialregister.nl [online], (2012).
Author information
Authors and Affiliations
Contributions
H. J. Lambers Heerspink and G. J. Navis wrote the article. H. J. Lambers Heerspink, M. H. de Borst, S. J. L. Bakker and G. J. Navis contributed to discussion of the content. M. H. de Borst and S. J. L. Bakker contributed to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lambers Heerspink, H., de Borst, M., Bakker, S. et al. Improving the efficacy of RAAS blockade in patients with chronic kidney disease. Nat Rev Nephrol 9, 112–121 (2013). https://doi.org/10.1038/nrneph.2012.281
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2012.281
This article is cited by
-
Echocardiographic manifestations in end-stage renal disease
Heart Failure Reviews (2023)
-
Angiotensin-[1–7] attenuates kidney injury in experimental Alport syndrome
Scientific Reports (2020)
-
Predictive factors for the development of proteinuria in cancer patients treated with bevacizumab, ramucirumab, and aflibercept: a single-institution retrospective analysis
Scientific Reports (2020)
-
Escaping residual albuminuria in hypertension: should we start eplerenone or reduce salt intake?
Hypertension Research (2019)
-
Evolving concepts in the pathogenesis of uraemic cardiomyopathy
Nature Reviews Nephrology (2019)