Abstract
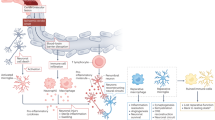
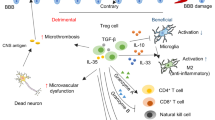
Recent clinical and experimental studies have highlighted a complex role for the immune system in the pathophysiological changes that occur after acute stroke. Sensors of the innate immune system such as Toll-like receptors, or effectors such as the lectin pathway of complement activation and innate immune cells, are activated by brain ischaemia and tissue damage, leading to amplification of the inflammatory cascade. Activation of the adaptive arm of the immune system, mediated by lymphocyte populations including T and B cells, regulatory T cells, and γδT cells, in response to stroke can lead to deleterious antigen-specific autoreactive responses but can also have cytoprotective effects. Increased incidence of infections is observed after acute stroke, and might result from activation of long-distance feedback loops between the CNS and peripheral immune organs, which are thought to play a part in stroke-induced immunodepression. Ongoing clinical trials are investigating whether the preventive use of antibiotics improves functional outcome after stroke. This Review discusses the multifaceted role of the immune system in the pathophysiology of acute stroke.
Key Points
-
Acute stroke is followed by a complex interaction between the brain and the immune system
-
Damage-associated molecular patterns are released after neuronal damage, and activate the innate and adaptive arms of the immune system
-
Different populations of lymphocytes can exert beneficial or detrimental functions after acute stroke, although the underlying mechanisms are not fully elucidated
-
Stroke can lead to immunodepression, increasing the risk of infections such as pneumonia
-
Ongoing studies are addressing the prophylactic use of antibiotics after stroke
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Engelhardt, B. & Ransohoff, R. M. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 26, 485–495 (2005).
Weller, R. O., Djuanda, E., Yow, H. Y. & Carare, R. O. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 117, 1–14 (2009).
Carson, M. J., Doose, J. M., Melchior, B., Schmid, C. D. & Ploix, C. C. CNS immune privilege: hiding in plain sight. Immunol. Rev. 213, 48–65 (2006).
Hanisch, U. K. & Kettenmann, H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 10, 1387–1394 (2007).
Bauer, J. et al. T-cell apoptosis in inflammatory brain lesions: destruction of T cells does not depend on antigen recognition. Am. J. Pathol. 153, 715–724 (1998).
Tian, L., Rauvala, H. & Gahmberg, C. G. Neuronal regulation of immune responses in the central nervous system. Trends Immunol. 30, 91–99 (2009).
Farina, C., Aloisi, F. & Meinl, E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 28, 138–145 (2007).
Perry, V. H. A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation. J. Neuroimmunol. 90, 113–121 (1998).
Steinman, L. Elaborate interactions between the immune and nervous systems. Nat. Immunol. 5, 575–581 (2004).
Galea, I., Bechmann, I. & Perry, V. H. What is immune privilege (not)? Trends Immunol. 8, 12–18 (2007).
Chen, G. Y. & Nuñez, G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826–837 (2010).
Schroeter, M., Jander, S., Witte, O. W. & Stoll, G. Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion. J. Neuroimmunol. 55, 195–203 (1994).
Tarkowski, E. et al. Early intrathecal production of interleukin-6 predicts the volume of brain lesion in stroke. Stroke 26, 1393–1398 (1995).
Fassbender, K. et al. Proinflammatory cytokines in serum of patients with acute cerebral ischemia: kinetics of secretion and relation to the extent of brain damage and outcome of disease. J. Neurol. Sci. 122, 135–139 (1994).
Beamer, N. B., Coull, B. M., Clark, W. M., Hazel, J. S. & Silberger, J. R. Interleukin-6 and interleukin-1 receptor antagonist in acute stroke. Ann. Neurol. 37, 800–804 (1995).
Chamorro, A., Urra, X. & Planas, A. M. Infection after acute ischemic stroke: a manifestation of brain-induced immunodepression. Stroke 38, 1097–1103 (2007).
Vila, N., Castillo, J., Dávalos, A. & Chamorro, A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke 31, 2325–2329 (2000).
Vila, N. et al. Levels of anti-inflammatory cytokines and neurological worsening in acute ischemic stroke. Stroke 34, 671–675 (2003).
Rubartelli, A. & Lotze, M. T. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 28, 429–436 (2007).
Pasare, C. & Medzhitov, R. Toll-like receptors: balancing host resistance with immune tolerance. Curr. Opin. Immunol. 15, 677–682 (2003).
Iadecola, C. & Anrather, J. The immunology of stroke: from mechanisms to translation. Nat. Med. 17, 796–808 (2011).
Lotze, M. T. & Tracey, K. J. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev.Immunol. 5, 331–342 (2005).
Goldstein, R. S. et al. Elevated high-mobility group box 1 levels in patients with cerebral and myocardial ischemia. Shock 25, 571–574 (2006).
Kono, H., Chen, C. J., Ontiveros, F. & Rock, K. L. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J. Clin. Invest. 120, 1939–1949 (2010).
Quintana, F. J. & Cohen, I. R. Heat shock proteins as endogenous adjuvants in sterile and septic inflammation. J. Immunol. 175, 2777–2782 (2005).
Bours, M. J., Swennen, E. L., Di Virgilio, F., Cronstein, B. N. & Dagnelie, P. C. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 112, 358–404 (2006).
Hofmann, M. A. et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell 97, 889–901 (1999).
Johnson, G. B., Brunn, G. J., Kodaira, Y. & Platt, J. L. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J. Immunol. 168, 5233–5239 (2002).
Burckstummer, T. et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 10, 266–272 (2009).
Kariko, K., Ni, H., Capodici, J., Lamphier, M. & Weissman, D. mRNA is an endogenous ligand for Toll-like receptor 3. J. Biol. Chem. 279, 12542–12550 (2004).
Kono, H. & Rock, K. L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 8, 279–289 (2008).
Kim, J. B. et al. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J. Neurosci. 26, 6413–6421 (2006).
Liu, K. et al. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J. 21, 3904–3916 (2007).
Hayakawa, K., Qiu, J. & Lo, E. H. Biphasic actions of HMGB1 signaling in inflammation and recovery after stroke. Ann. NY Acad. Sci. 1207, 50–57 (2010).
Romanos, E., Planas, A. M., Amaro, S. & Chamorro, A. Uric acid reduces brain damage and improves the benefits of rt-PA in a rat model of thromboembolic stroke. J. Cereb. Blood Flow Metab. 27, 14–20 (2007).
Amaro, S. et al. Uric acid levels are relevant in patients with stroke treated with thrombolysis. Stroke 42 (1 Suppl.), S28–S32 (2011).
Hanke, M. L. & Kielian, T. Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin. Sci. (Lond.) 121, 367–387 (2011).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Lehnardt, S. et al. Toll-like receptor 2 mediates CNS injury in focal cerebral ischemia. J. Neuroimmunol. 190, 28–33 (2007).
Caso, J. R. et al. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation 115, 1599–1608 (2007).
Sansing, L. H. et al. Toll-like receptor 4 contributes to poor outcome after intracerebral hemorrhage. Ann. Neurol. 70, 646–656 (2011).
Urra, X. et al. Monocyte subtypes predict clinical course and prognosis in human stroke. J. Cereb. Blood Flow Metab. 29, 994–1002 (2009).
Geissmann, F. et al. Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 (2010).
Garcia, J. H. et al. Influx of leukocytes and platelets in an evolving brain infarct (Wistar rat). Am. J. Pathol. 144, 188–199 (1994).
Urra, X. et al. Monocytes are major players in the prognosis and risk of infection after acute stroke. Stroke 40, 1262–1268 (2009).
Haeusler, K. G. et al. Cellular immunodepression preceding infectious complications after acute ischemic stroke in humans. Cerebrovasc. Dis. 25, 50–58 (2008).
Harms, H. et al. Preventive antibacterial therapy in acute ischemic stroke: a randomized controlled trial. PLoS ONE 3, e2158 (2008).
Klehmet, J. et al. Stroke-induced immunodepression and post-stroke infections: lessons from the Preventive Antibacterial Therapy in Stroke trial. Neuroscience 158, 1184–1193 (2009).
Vogelgesang, A. et al. Analysis of lymphocyte subsets in patients with stroke and their influence on infection after stroke. Stroke 39, 237–241 (2008).
Hug, A. et al. Infarct volume is a major determiner of post-stroke immune cell function and susceptibility to infection. Stroke 40, 3226–3232 (2009).
Yang, Q. W. et al. Upregulated expression of toll-like receptor 4 in monocytes correlates with severity of acute cerebral infarction. J. Cereb. Blood Flow Metab. 28, 1588–1596 (2008).
Mocco, J. et al. Alterations in plasma complement levels after human ischemic stroke. Neurosurgery 59, 28–33 (2006).
Lindsberg, P. J. et al. Complement activation in the central nervous system following blood-brain barrier damage in man. Ann. Neurol. 40, 587–596 (1996).
Cervera, A. et al. Genetically-defined deficiency of mannose-binding lectin is associated with protection after experimental stroke in mice and outcome in human stroke. PLoS ONE 5, e8433 (2010).
Yilmaz, G., Arumugam, T. V., Stokes, K. Y. & Granger, D. N. Role of T lymphocytes and interferon-γ in ischemic stroke. Circulation 113, 2105–2112 (2006).
Shichita, T. et al. Pivotal role of cerebral interleukin-17-producing γδT cells in the delayed phase of ischemic brain injury. Nat. Med. 15, 946–950 (2009).
Liesz, A. et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat. Med. 15, 192–199 (2009).
Kleinschnitz, C. et al. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood 115, 3835–3842 (2010).
Liesz, A. et al. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain 134, 704–720 (2011).
Gelderblom, M. et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 40, 1849–1857 (2009).
Engelhardt, B. & Sorokin, L. The blood–brain and the blood–cerebrospinal fluid barriers: function and dysfunction. Semin. Immunopathol. 31, 497–511 (2009).
Martin, B., Hirota, K., Cua, D. J., Stockinger, B. & Veldhoen, M. Interleukin-17-producing γδT cells selectively expand in response to pathogen products and environmental signals. Immunity 31, 321–330 (2009).
Konoeda, F. et al. Therapeutic effect of IL-12/23 and their signaling pathway blockade on brain ischemia model. Biochem. Biophys. Res. Commun. 402, 500–506 (2010).
Czech, B. et al. The immunomodulatory sphingosine 1-phosphate analog FTY720 reduces lesion size and improves neurological outcome in a mouse model of cerebral ischemia. Biochem. Biophys. Res. Commun. 389, 251–256 (2009).
Wei, Y. et al. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann. Neurol. 69, 119–129 (2011).
Liesz, A. et al. FTY720 reduces post-ischemic brain lymphocyte influx but does not improve outcome in permanent murine cerebral ischemia. PLoS ONE 6, e21312 (2011).
Wang, W. Z. et al. Myelin antigen reactive T cells in cerebrovascular diseases. Clin. Exp. Immunol. 88, 157–162 (1992).
Bornstein, N. M. et al. Antibodies to brain antigens following stroke. Neurology 56, 529–530 (2001).
Planas, A. M. et al. Brain-derived antigens in lymphoid tissue of patients with acute stroke. J. Immunol. 188, 2156–2163 (2012).
Becker, K. J. Sensitization and tolerization to brain antigens in stroke. Neuroscience 158, 1090–1097 (2009).
Becker, K. J., Kindrick, D. L., Lester, M. P., Shea, C. & Ye, Z. C. Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. J. Cereb. Blood Flow Metab. 25, 1634–1644 (2005).
Becker, K. J. et al. Autoimmune responses to the brain after stroke are associated with worse outcome. Stroke 42, 2763–2769 (2011).
Subramanian, S. et al. Recombinant T cell receptor ligand treats experimental stroke. Stroke 40, 2539–2545 (2009).
Moalem, G. et al. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat. Med. 5, 49–55 (1999).
Lewitus, G. M., Kipnis, J., Avidan, H., Ben-Nun, A. & Schwartz, M. Neuroprotection induced by mucosal tolerance is epitope-dependent: conflicting effects in different strains. J. Neuroimmunol. 175, 31–38 (2006).
Ziv, Y. et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 9, 268–275 (2006).
Frenkel, D. et al. Nasal vaccination with myelin oligodendrocyte glycoprotein reduces stroke size by inducing IL-10-producing CD4+ T cells. J. Immunol. 171, 6549–6555 (2003).
Gee, J. M., Kalil, A., Thullbery, M. & Becker, K. J. Induction of immunologic tolerance to myelin basic protein prevents central nervous system autoimmunity and improves outcome after stroke. Stroke 39, 1575–1582 (2008).
Takeda, H. et al. Induction of mucosal tolerance to E-selectin prevents ischemic and hemorrhagic stroke in spontaneously hypertensive genetically stroke-prone rats. Stroke 33, 2156–2163 (2002).
Chen, Y. et al. Mucosal tolerance to E-selectin provides cell-mediated protection against ischemic brain injury. Proc. Natl Acad. Sci. USA 100, 15107–15112 (2003).
Hallenbeck, J. How inflammation modulates central nervous system vessel activation and provides targets for intervention—a personal perspective. Ann. NY Acad. Sci. 1207, 1–7 (2010).
Gee, J. M. et al. Long term immunologic consequences of experimental stroke and mucosal tolerance. Exp. Transl. Stroke Med. 1, 3 (2009).
Sakaguchi, S., Yamaguchi, T., Nomura, T. & Ono, M. Regulatory T cells and immune tolerance. Cell 133, 775–787 (2008).
Offner, H. et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J. Immunol. 176, 6523–6531 (2006).
Ren, X., Akiyosji, K., Vandenbark, A. A., Hurn, P. D. & Offner, H. CD4+FoxP3+ regulatory T-cells in cerebral ischemic stroke. Metab. Brain Dis. 26, 87–90 (2011).
Ooboshi, H. et al. Postischemic gene transfer of interleukin-10 protects against both focal and global brain ischemia. Circulation 2, 913–919 (2005).
Ren, X. et al. Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. J. Neurosci. 31, 8556–8563 (2011).
Justicia, C. et al. Neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/reperfusion of the middle cerebral artery in rats. J. Cereb. Blood Flow Metab. 23, 1430–1440 (2003).
Mills, K. H. TLR-dependent T cell activation in autoimmunity. Nat. Rev. Immunol. 11, 807–822 (2011).
Lake, J., Weller, R. O., Phillips, M. J. & Needham, M. Lymphocyte targeting of the brain in adoptive transfer cryolesion-EAE. J. Pathol. 187, 259–265 (1999).
Westendorp, W. F., Nederkoorn, P. J., Vermeij, J. D., Dijkgraaf, M. G. & van de Beek, D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. 11, 110 (2011).
Finlayson, O. et al. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology 77, 1338–1345 (2011).
Katzan, I. L., Dawson, N. V., Thomas, C. L., Votruba, M. E. & Cebul, R. D. The cost of pneumonia after acute stroke. Neurology 68, 1938–1943 (2007).
Katzan, I. L., Cebul, R. D., Husak, S. H., Dawson, N. V. & Baker, D. W. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology 60, 620–625 (2003).
van de Beek, D. et al. Preventive antibiotics for infections in acute stroke: a systematic review and meta-analysis. Arch. Neurol. 66, 1076–1081 (2009).
Martino, R. et al. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke 36, 2756–2763 (2005).
Sellars, C. et al. Risk factors for chest infection in acute stroke: a prospective cohort study. Stroke 38, 2284–2291 (2007).
Westendorp, W. F. et al. Antibiotic therapy for preventing infections in patients with acute stroke. Cochrane Database of Systematic Reviews, Issue 1. Art. No.: CD008530. http://dx.doi.org/10.1002/14651858.CD008530.pub2.
Poisson, S. N., Johnston, S. C. & Josephson, S. A. Urinary tract infections complicating stroke: mechanisms, consequences, and possible solutions. Stroke 41, e180–e184 (2010).
Stott, D. J., Falconer, A., Miller, H., Tilston, J. C. & Langhorne, P. Urinary tract infection after stroke. QJM 102, 243–249 (2009).
Wrona, D. Neural–immune interactions: an integrative view of the bidirectional relationship between the brain and immune systems. J. Neuroimmunol. 172, 38–58 (2006).
Tarkowski, E., Naver, H., Wallin, B. G., Blomstrand, C. & Tarkowski, A. Lateralization of T-lymphocyte responses in patients with stroke. Effect of sympathetic dysfunction? Stroke 26, 57–62 (1995).
Gendron, A. et al. Temporal effects of left versus right middle cerebral artery occlusion on spleen lymphocyte subsets and mitogenic response in Wistar rats. Brain Res. 955, 85–97 (2002).
Harms, H. et al. Influence of stroke localization on autonomic activation, immunodepression, and post-stroke infection. Cerebrovasc. Dis. 32, 552–560 (2011).
Steinhagen, V., Grossmann, A., Benecke, R. & Walter, U. Swallowing disturbance pattern relates to brain lesion location in acute stroke patients. Stroke 40, 1903–1906 (2009).
Minnerup, J. et al. The impact of lesion location and lesion size on poststroke infection frequency. J. Neurol. Neurosurg. Psychiatry 81, 198–202 (2010).
Liesz, A. et al. The spectrum of systemic immune alterations after murine focal ischemia: immunodepression versus immunomodulation. Stroke 40, 2849–2858 (2009).
Millns, B., Gosney, M., Jack, C. I., Martin, M. V. & Wright, A. E. Acute stroke predisposes to oral gram-negative bacilli—a cause of aspiration pneumonia? Gerontology 49, 173–176 (2003).
Ersoz, M., Ulusoy, H., Oktar, M. A. & Akyuz, M. Urinary tract infection and bacteriurua in stroke patients: frequencies, pathogen microorganisms, and risk factors. Am. J. Phys. Med. Rehabil. 86, 734–741 (2007).
Prass, K. et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J. Exp. Med. 198, 725–736 (2003).
Meisel, C., Schwab, J. M., Prass, K., Meisel, A. & Dirnagl, U. Central nervous system injury-induced immune deficiency syndrome. Nat. Rev. Neurosci. 6, 775–786 (2005).
Wong, C. H., Jenne, C. N., Lee, W. Y., Léger, C. & Kubes, P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science 334, 101–105 (2011).
Rosas-Ballina, M. et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101 (2011).
Trakhtenberg, E. F. & Goldberg, J. L. Neuroimmune communication. Science 334, 47–48 (2011).
Hug, A. et al. Reduced efficacy of circulating costimulatory cells after focal cerebral ischemia. Stroke 42, 3580–3586 (2011).
Vogelgesang, A. et al. Functional status of peripheral blood T-cells in ischemic stroke patients. PLoS ONE 5, e8718 (2010).
Chamorro, A. et al. Catecholamines, infection, and death in acute ischemic stroke. J. Neurol. Sci. 252, 29–35 (2007).
Chamorro, A. et al. Interleukin 10, monocytes and increased risk of early infection in ischemic stroke. J. Neurol. Neurosurg. Psychiatry 77, 1279–1281 (2006).
Woiciechowsky, C., Schöning, B., Lanksch, W. R., Volk, H. D. & Döcke, W. D. Mechanisms of brain-mediated systemic anti-inflammatory syndrome causing immunodepression. J. Mol. Med. 77, 769–780 (1999).
Schaller, B. J., Graf, R. & Jacobs, A. H. Pathophysiological changes of the gastrointestinal tract in ischemic stroke. Am. J. Gastroenterol. 101, 1655–1665 (2006).
De Falco, F. A., Santangelo, R., Majello, L. & Angelone, P. Antimicrobial prophylaxis in the management of ischemic stroke [Italian]. Riv. Neurobiol. 44, 63–77 (1998).
Chamorro, A. et al. The Early Systemic Prophylaxis of Infection After Stroke study: a randomized clinical trial. Stroke 36, 1495–1500 (2005).
Lampl, Y. et al. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology 69, 1404–1410 (2007).
Schwarz, S., Al-Shajlawi, F., Sick, C., Meairs, S. & Hennerici, M. G. Effects of prophylactic antibiotic therapy with mezlocillin plus sulbactam on the incidence and height of fever after severe acute ischemic stroke: the Mannheim infection in stroke study (MISS). Stroke 39, 1220–1227 (2008).
Nederkoorn, P. J. et al. Preventive antibiotics in stroke study: rationale and protocol for randomised trial. Int. J. Stroke 6, 159–163 (2011).
Rothstein, J. D. et al. β-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 433, 73–77 (2005).
Lee, H., Park, J. W., Kim, S. P., Lo, E. H. & Lee, S. R. Doxycycline inhibits matrix metalloproteinase-9 and laminin degradation after transient global cerebral ischemia. Neurobiol. Dis. 34, 189–198 (2009).
Fagan, S. C. et al. Minocycline to improve neurologic outcome in stroke (MINOS): a dose-finding study. Stroke 41, 2283–2287 (2010).
Lee, H., Park, J. W., Kim, S. P., Lo, E. H. & Lee, S. R. Doxycycline inhibits matrix metalloproteinase-9 and laminin degradation after transient global cerebral ischemia. Neurobiol. Dis. 34, 189–198 (2009).
O'Collins, V. E. et al. Preclinical drug evaluation for combination therapy in acute stroke using systematic review, meta-analysis, and subsequent experimental testing. J. Cereb. Blood Flow Metab. 31, 962–975 (2011).
Acknowledgements
This Review is dedicated to the memory of Dr Jesús Chamorro (1922–2012), who died during the preparation of the manuscript. This work was supported in part by a grant awarded to Ángel Chamorro from the Melchor Collet Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data for the article, discussion of the content, writing the article, and to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Chamorro, Á., Meisel, A., Planas, A. et al. The immunology of acute stroke. Nat Rev Neurol 8, 401–410 (2012). https://doi.org/10.1038/nrneurol.2012.98
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2012.98
This article is cited by
-
Stress hyperglycemia increases short-term mortality in acute ischemic stroke patients after mechanical thrombectomy
Diabetology & Metabolic Syndrome (2024)
-
Pathogenesis-adaptive polydopamine nanosystem for sequential therapy of ischemic stroke
Nature Communications (2023)
-
Blood inflammatory biomarkers predict in-hospital pneumonia after endovascular treatment of aneurysm in patients with aneurysmal subarachoid hemorrhage
Neurosurgical Review (2023)
-
Recombinant human brain natriuretic peptide attenuates ischemic brain injury in mice by inhibiting oxidative stress and cell apoptosis via activation of PI3K/AKT/Nrf2/HO-1 pathway
Experimental Brain Research (2023)
-
Reinventing the Penumbra — the Emerging Clockwork of a Multi-modal Mechanistic Paradigm
Translational Stroke Research (2023)