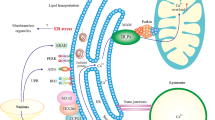
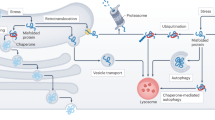
Key Points
-
Many neurodegenerative diseases involve the accumulation of protein aggregates
-
Endoplasmic reticulum (ER) stress triggers activation of the unfolded protein response (UPR), an adaptive reaction that restores cellular protein homeostasis, known as proteostasis
-
Dysfunction of proteostasis is associated with abnormal levels of ER stress and is associated with neuronal degeneration in human post-mortem brain tissue
-
Targeting the UPR can have distinct and even opposite effects on disease progression, depending on the disease context and the signalling branch that is analysed
-
Gene therapy and pharmacological strategies to attenuate ER stress alleviates degeneration in various disease models
-
Chronic ER stress not only results in neuronal loss, but also represses the synthesis of synaptic proteins, with implications for cognition and memory, and possibly autism spectrum disorder
Abstract
The clinical manifestation of neurodegenerative diseases is initiated by the selective alteration in the functionality of distinct neuronal populations. The pathology of many neurodegenerative diseases includes accumulation of misfolded proteins in the brain. In physiological conditions, the proteostasis network maintains normal protein folding, trafficking and degradation; alterations in this network — particularly disturbances to the function of endoplasmic reticulum (ER) — are thought to contribute to abnormal protein aggregation. ER stress triggers a signalling reaction known as the unfolded protein response (UPR), which induces adaptive programmes that improve protein folding and promote quality control mechanisms and degradative pathways or can activate apoptosis when damage is irreversible. In this Review, we discuss the latest advances in defining the functional contribution of ER stress to brain diseases, including novel evidence that relates the UPR to synaptic function, which has implications for cognition and memory. A complex concept is emerging wherein the consequences of ER stress can differ drastically depending on the disease context and the UPR signalling pathway that is altered. Strategies to target specific components of the UPR using small molecules and gene therapy are in development, and promise interesting avenues for future interventions to delay or stop neurodegeneration.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Soto, C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat. Rev. Neurosci. 4, 49–60 (2003).
Aguzzi, A. & O'Connor, T. Protein aggregation diseases: pathogenicity and therapeutic perspectives. Nat. Rev. Drug Discov. 9, 237–248 (2010).
Bertram, L. & Tanzi, R. E. The genetic epidemiology of neurodegenerative disease. J. Clin. Invest. 115, 1449–1457 (2005).
Taylor, J. P., Hardy, J. & Fischbeck, K. H. Toxic proteins in neurodegenerative disease. Science 296, 1991–1995 (2002).
Kaushik, S. & Cuervo, A. M. Proteostasis and aging. Nat. Med. 21, 1406–1415 (2015).
Balch, W. E., Morimoto, R. I., Dillin, A. & Kelly, J. W. Adapting proteostasis for disease intervention. Science 319, 916–919 (2008).
Hetz, C. & Mollereau, B. Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat. Rev. Neurosci. 15, 233–249 (2014).
Frake, R. A., Ricketts, T., Menzies, F. M. & Rubinsztein, D. C. Autophagy and neurodegeneration. J. Clin. Invest. 125, 65–74 (2015).
Wang, M. & Kaufman, R. J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 529, 326–335 (2016).
Oakes, S. A. & Papa, F. R. The role of endoplasmic reticulum stress in human pathology. Annu. Rev. Pathol. 10, 173–194 (2015).
Martinez, G., Duran-Aniotz, C., Cabral-Miranda, F., Vivar, J. P. & Hetz, C. Endoplasmic reticulum proteostasis impairment in aging. Aging Cell http://dx/doi.org/10.1111/acel.12599 (2017).
Cornejo, V. H., Pihan, P., Vidal, R. L. & Hetz, C. Role of the unfolded protein response in organ physiology: lessons from mouse models. IUBMB Life 65, 962–975 (2013).
Chow, C. Y., Wang, X., Riccardi, D., Wolfner, M. F. & Clark, A. G. The genetic architecture of the genome-wide transcriptional response to ER stress in the mouse. PLoS Genet. 11, e1004924 (2015).
Hetz, C., Chevet, E. & Oakes, S. A. Proteostasis control by the unfolded protein response. Nat. Cell Biol. 17, 829–838 (2015).
Dombroski, B. A. et al. Gene expression and genetic variation in response to endoplasmic reticulum stress in human cells. Am. J. Hum. Genet. 86, 719–729 (2010).
Walter, P. & Ron, D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 (2011).
Maurel, M., Chevet, E., Tavernier, J. & Gerlo, S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem. Sci. 39, 245–254 (2014).
Maly, D. J. & Papa, F. R. Druggable sensors of the unfolded protein response. Nat. Chem. Biol. 10, 892–901 (2014).
Hetz, C., Chevet, E. & Harding, H. P. Targeting the unfolded protein response in disease. Nat. Rev. Drug Discov. 12, 703–719 (2013).
Pakos-Zebrucka, K. et al. The integrated stress response. EMBO Rep. 17, 1374–1395 (2016).
Chafekar, S. M. et al. Increased Aβ1-42 production sensitizes neuroblastoma cells for ER stress toxicity. Curr. Alzheimer Res. 5, 469–474 (2008).
Cornejo, V. H. & Hetz, C. The unfolded protein response in Alzheimer's disease. Semin. Immunopathol. 35, 277–292 (2013).
Gouras, G. K. et al. Intraneuronal Aβ42 accumulation in human brain. Am. J. Pathol. 156, 15–20 (2000).
Fernandez-Vizarra, P. et al. Intra- and extracellular Aβ and PHF in clinically evaluated cases of Alzheimer's disease. Histol. Histopathol. 19, 823–844 (2004).
Hetz, C., Russelakis-Carneiro, M., Maundrell, K., Castilla, J. & Soto, C. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 22, 5435–5445 (2003). The first paper to describe ER stress in tissue from a patient with neurodegeneration.
Torres, M. et al. Prion protein misfolding affects calcium homeostasis and sensitizes cells to endoplasmic reticulum stress. PLoS ONE 5, e15658 (2010).
Ferreiro, E. et al. Involvement of mitochondria in endoplasmic reticulum stress-induced apoptotic cell death pathway triggered by the prion peptide PrP(106–126). J. Neurochem. 104, 766–776 (2008).
Coe, H. & Michalak, M. Calcium binding chaperones of the endoplasmic reticulum. Gen. Physiol. Biophys. 28, F96–F103 (2009).
Katayama, T. et al. Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nat. Cell Biol. 1, 479–485 (1999).
Terro, F. et al. Neurons overexpressing mutant presenilin-1 are more sensitive to apoptosis induced by endoplasmic reticulum-Golgi stress. J. Neurosci. Res. 69, 530–539 (2002).
Piccini, A. et al. Fibroblasts from FAD-linked presenilin 1 mutations display a normal unfolded protein response but overproduce Aβ42 in response to tunicamycin. Neurobiol. Dis. 15, 380–386 (2004).
Steiner, H., Winkler, E., Shearman, M. S., Prywes, R. & Haass, C. Endoproteolysis of the ER stress transducer ATF6 in the presence of functionally inactive presenilins. Neurobiol. Dis. 8, 717–722 (2001).
Meier, S. et al. Identification of novel Tau interactions with endoplasmic reticulum proteins in Alzheimer's disease brain. J. Alzheimers Dis. 48, 687–702 (2015).
Abisambra, J. F. et al. Tau accumulation activates the unfolded protein response by impairing endoplasmic reticulum-associated degradation. J. Neurosci. 33, 9498–9507 (2013).
Bellucci, A. et al. Induction of the unfolded protein response by α-synuclein in experimental models of Parkinson's disease. J. Neurochem. 116, 588–605 (2011).
Cooper, A. A. et al. α-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science 313, 324–328 (2006). Study showing that the earliest deficit that results from α-synuclein expression is a block of trafficking from the ER to the Golgi apparatus, explaining the occurrence of ER stress in PD.
Credle, J. J. et al. α-Synuclein-mediated inhibition of ATF6 processing into COPII vesicles disrupts UPR signaling in Parkinson's disease. Neurobiol. Dis. 76, 112–125 (2015).
Takahashi, R., Imai, Y., Hattori, N. & Mizuno, Y. Parkin and endoplasmic reticulum stress. Ann. NY Acad. Sci. 991, 101–106 (2003).
Imai, Y. et al. An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell 105, 891–902 (2001).
Ugolino, J., Fang, S., Kubisch, C. & Monteiro, M. J. Mutant Atp13a2 proteins involved in parkinsonism are degraded by ER-associated degradation and sensitize cells to ER-stress induced cell death. Hum. Mol. Genet. 20, 3565–3577 (2011).
Kikuchi, H. et al. Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an ALS model. Proc. Natl Acad. Sci. USA 103, 6025–6030 (2006).
Nishitoh, H. et al. ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting Derlin-1. Genes Dev. 22, 1451–1464 (2008). The first study to show that mutant SOD1 triggers ER stress by interacting with and blocking activity of the ERAD component Derlin-1 in ALS.
Urushitani, M., Ezzi, S. A., Matsuo, A., Tooyama, I. & Julien, J. P. The endoplasmic reticulum-Golgi pathway is a target for translocation and aggregation of mutant superoxide dismutase linked to ALS. Faseb J. 22, 2476–2487 (2008).
Atkin, J. D. et al. Induction of the unfolded protein response in familial amyotrophic lateral sclerosis and association of protein-disulfide isomerase with superoxide dismutase 1. J. Biol. Chem. 281, 30152–30165 (2006).
Farg, M. A. et al. Ataxin-2 interacts with FUS and intermediate-length polyglutamine expansions enhance FUS-related pathology in amyotrophic lateral sclerosis. Hum. Mol. Genet. 22, 717–728 (2013).
Farg, M. A. et al. Mutant FUS induces endoplasmic reticulum stress in amyotrophic lateral sclerosis and interacts with protein disulfide-isomerase. Neurobiol. Aging 33, 2855–2868 (2012).
Walker, A. K. et al. Protein disulphide isomerase protects against protein aggregation and is S-nitrosylated in amyotrophic lateral sclerosis. Brain 133, 105–116 (2010).
Uehara, T. et al. S-Nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 441, 513–517 (2006).
Gkogkas, C. et al. VAPB interacts with and modulates the activity of ATF6. Hum. Mol. Genet. 17, 1517–1526 (2008).
Zhang, Y. J. et al. Aggregation-prone c9FTD/ALS poly(GA) RAN-translated proteins cause neurotoxicity by inducing ER stress. Acta Neuropathol. 128, 505–524 (2014).
Duennwald, M. L. & Lindquist, S. Impaired ERAD and ER stress are early and specific events in polyglutamine toxicity. Genes Dev. 22, 3308–3319 (2008).
Yang, H. et al. Huntingtin interacts with the cue domain of gp78 and inhibits gp78 binding to ubiquitin and p97/VCP. PLoS ONE 5, e8905 (2010).
El-Daher, M. T. et al. Huntingtin proteolysis releases non-polyQ fragments that cause toxicity through dynamin 1 dysregulation. EMBO J. 34, 2255–2271 (2015).
Freeman, O. J. & Mallucci, G. R. The UPR and synaptic dysfunction in neurodegeneration. Brain Res. 1648, 530–537 (2016).
Hoozemans, J. J. et al. The unfolded protein response is activated in pretangle neurons in Alzheimer's disease hippocampus. Am. J. Pathol. 174, 1241–1251 (2009).
Hoozemans, J. J. et al. The unfolded protein response is activated in Alzheimer's disease. Acta Neuropathol. 110, 165–172 (2005).
Stutzbach, L. D. et al. The unfolded protein response is activated in disease-affected brain regions in progressive supranuclear palsy and Alzheimer's disease. Acta Neuropathol. Commun. 1, 31 (2013).
Unterberger, U. et al. Endoplasmic reticulum stress features are prominent in Alzheimer disease but not in prion diseases in vivo. J. Neuropathol. Exp. Neurol. 65, 348–357 (2006).
Duran-Aniotz, C. et al. IRE1 signaling exacerbates Alzheimer's disease pathogenesis. Acta Neuropathol. http://dx.doi.org/10.1007/s00401-017-1694-x (2017). The first report to show that IRE1α signaling is pathogenic in Alzheimer disease mouse models.
Nijholt, D. A., van Haastert, E. S., Rozemuller, A. J., Scheper, W. & Hoozemans, J. J. The unfolded protein response is associated with early tau pathology in the hippocampus of tauopathies. J. Pathol. 226, 693–702 (2012).
Hoozemans, J. J. et al. Activation of the unfolded protein response in Parkinson's disease. Biochem. Biophys. Res. Commun. 354, 707–711 (2007).
Hoozemans, J. J., van Haastert, E. S., Nijholt, D. A., Rozemuller, A. J. & Scheper, W. Activation of the unfolded protein response is an early event in Alzheimer's and Parkinson's disease. Neurodegener Dis. 10, 212–215 (2012).
Makioka, K. et al. Involvement of endoplasmic reticulum stress defined by activated unfolded protein response in multiple system atrophy. J. Neurol. Sci. 297, 60–65 (2010).
Atkin, J. D. et al. Endoplasmic reticulum stress and induction of the unfolded protein response in human sporadic amyotrophic lateral sclerosis. Neurobiol. Dis. 30, 400–407 (2008).
Hetz, C. et al. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 23, 2294–2306 (2009). This study showed that inhibition of XBP1 signalling in ALS ameliorates disease pathology through cross-talk with ER stress and autophagy in vivo.
Ito, Y. et al. Involvement of CHOP, an ER-stress apoptotic mediator, in both human sporadic ALS and ALS model mice. Neurobiol. Dis. 36, 470–476 (2009).
Sasaki, S. Endoplasmic reticulum stress in motor neurons of the spinal cord in sporadic amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 69, 346–355 (2010).
Ilieva, E. V. et al. Oxidative and endoplasmic reticulum stress interplay in sporadic amyotrophic lateral sclerosis. Brain 130, 3111–3123 (2007).
Nardo, G. et al. Amyotrophic lateral sclerosis multiprotein biomarkers in peripheral blood mononuclear cells. PLoS ONE 6, e25545 (2011).
Prudencio, M. et al. Distinct brain transcriptome profiles in C9orf72-associated and sporadic ALS. Nat. Neurosci. 18, 1175–1182 (2015).
Yoo, B. C. et al. Overexpressed protein disulfide isomerase in brains of patients with sporadic Creutzfeldt-Jakob disease. Neurosci. Lett. 334, 196–200 (2002).
Wiersma, V. I. et al. Activation of the unfolded protein response and granulovacuolar degeneration are not common features of human prion pathology. Acta Neuropathol. Commun. 4, 113 (2016).
Carnemolla, A. et al. Rrs1 is involved in endoplasmic reticulum stress response in Huntington disease. J. Biol. Chem. 284, 18167–18173 (2009).
Kalathur, R. K. et al. The unfolded protein response and its potential role in Huntington's disease elucidated by a systems biology approach. F1000Res 4, 103 (2015).
Vidal, R. L. et al. Targeting the UPR transcription factor XBP1 protects against Huntington's disease through the regulation of FoxO1 and autophagy. Hum. Mol. Genet. 21, 2245–2262 (2012).
Chung, C. Y. et al. Identification and rescue of α-synuclein toxicity in Parkinson patient-derived neurons. Science 342, 983–987 (2013). This study showed that cortical neurons derived from iPSCs from patients with Parkinson disease exhibit nitrosative stress and accumulation of ERAD substrates, which trigger ER stress.
Fernandes, H. J. et al. ER stress and autophagic perturbations lead to elevated extracellular α-synuclein in GBA-N370S Parkinson's iPSC-derived dopamine neurons. Stem Cell Rep. 6, 342–356 (2016).
Kiskinis, E. et al. Pathways disrupted in human ALS motor neurons identified through genetic correction of mutant SOD1. Cell Stem Cell 14, 781–795 (2014). The first study to show that ER stress is a common pathological response in motor neurons derived from rodents and patients with ALS.
Dafinca, R. et al. C9orf72 hexanucleotide expansions are associated with altered endoplasmic reticulum calcium homeostasis and stress granule formation in induced pluripotent stem cell-derived neurons from patients with amyotrophic lateral sclerosis and frontotemporal dementia. Stem Cells 34, 2063–2078 (2016).
Hall, C. E. et al. Progressive motor neuron pathology and the role of astrocytes in a human stem cell model of VCP-related ALS. Cell Rep. 19, 1739–1749 (2017).
Nekrasov, E. D. et al. Manifestation of Huntington's disease pathology in human induced pluripotent stem cell-derived neurons. Mol. Neurodegener. 11, 27 (2016).
Kondo, T. et al. Modeling Alzheimer's disease with iPSCs reveals stress phenotypes associated with intracellular Aβ and differential drug responsiveness. Cell Stem Cell 12, 487–496 (2013).
Jin, Z. B., Okamoto, S., Xiang, P. & Takahashi, M. Integration-free induced pluripotent stem cells derived from retinitis pigmentosa patient for disease modeling. Stem Cells Transl. Med. 1, 503–509 (2012).
Tucker, B. A. et al. Patient-specific iPSC-derived photoreceptor precursor cells as a means to investigate retinitis pigmentosa. eLife 2, e00824 (2013).
Saxena, S. & Caroni, P. Selective neuronal vulnerability in neurodegenerative diseases: from stressor thresholds to degeneration. Neuron 71, 35–48 (2011).
Scheper, W. & Hoozemans, J. J. The unfolded protein response in neurodegenerative diseases: a neuropathological perspective. Acta Neuropathol. 130, 315–331 (2015).
Axten, J. M. et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (GSK2656157), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). J. Med. Chem. 23, 7193–7207 (2013).
Boyce, M. et al. A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science 307, 935–939 (2005).
Tsaytler, P., Harding, H. P., Ron, D. & Bertolotti, A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science 332, 91–94 (2011). This report identified guanabenz as a potential drug to target the integrated stress response for therapy in protein misfolding disorders.
Das, I. et al. Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science 348, 239–242 (2015).
Sidrauski, C. et al. Pharmacological brake-release of mRNA translation enhances cognitive memory. eLife 2, e00498 (2013). This study identified the small molecule integrated stress response inhibitor (ISRIB), its targeting of the integrated stress response, and its role in memory consolidation.
Sidrauski, C. et al. Pharmacological dimerization and activation of the exchange factor eIF2B antagonizes the integrated stress response. eLife 4, e07314 (2015).
Wang, L., Popko, B. & Roos, R. P. The unfolded protein response in familial amyotrophic lateral sclerosis. Hum. Mol. Genet. 20, 1008–1015 (2011).
Wang, L., Popko, B. & Roos, R. P. An enhanced integrated stress response ameliorates mutant SOD1-induced ALS. Hum. Mol. Genet. 23, 2629–2638 (2014).
Wang, L., Popko, B., Tixier, E. & Roos, R. P. Guanabenz, which enhances the unfolded protein response, ameliorates mutant SOD1-induced amyotrophic lateral sclerosis. Neurobiol. Dis. 71, 317–324 (2014).
Jiang, H. Q. et al. Guanabenz delays the onset of disease symptoms, extends lifespan, improves motor performance and attenuates motor neuron loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neuroscience 277, 132–138 (2014).
Saxena, S., Cabuy, E. & Caroni, P. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat. Neurosci. 12, 627–636 (2009). This study showed that ER stress is the earliest defect in models of ALS, and that this defect determines the selective vulnerability of motor neurons in ALS.
Vaccaro, A. et al. Pharmacological reduction of ER stress protects against TDP-43 neuronal toxicity in vivo. Neurobiol. Dis. 55, 64–75 (2013).
Vieira, F. G. et al. Guanabenz treatment accelerates disease in a mutant SOD1 mouse model of ALS. PLoS ONE 10, e0135570 (2015).
Matus, S., Lopez, E., Valenzuela, V., Nassif, M. & Hetz, C. Functional contribution of the transcription factor ATF4 to the pathogenesis of amyotrophic lateral sclerosis. PLoS ONE 8, e66672 (2013).
Baleriola, J. et al. Axonally synthesized ATF4 transmits a neurodegenerative signal across brain regions. Cell 158, 1159–1172 (2014).
Silva, R. M. et al. CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J. Neurochem. 95, 974–986 (2005).
Colla, E. et al. Endoplasmic reticulum stress is important for the manifestations of α-synucleinopathy in vivo. J. Neurosci. 32, 3306–3320 (2012).
Pennuto, M. et al. Ablation of the UPR-mediator CHOP restores motor function and reduces demyelination in Charcot-Marie-Tooth 1B mice. Neuron 57, 393–405 (2008).
D'Antonio, M. et al. Resetting translational homeostasis restores myelination in Charcot-Marie-Tooth disease type 1B mice. J. Exp. Med. 210, 821–838 (2013).
Sidoli, M. et al. Ablation of Perk in Schwann cells improves Myelination in the S63del Charcot-Marie-Tooth 1B mouse. J. Neurosci. 36, 11350–11361 (2016).
Crespillo-Casado, A., Chambers, J. E., Fischer, P. M., Marciniak, S. J. & Ron, D. PPP1R15A-mediated dephosphorylation of eIF2α is unaffected by Sephin1 or Guanabenz. eLife 6, e26109 (2017).
Southwood, C. M., Garbern, J., Jiang, W. & Gow, A. The unfolded protein response modulates disease severity in Pelizaeus-Merzbacher disease. Neuron 36, 585–596 (2002).
Valenzuela, V., Martinez, G., Duran-Aniotz, C. & Hetz, C. Gene therapy to target ER stress in brain diseases. Brain Res. 1648, 561–570 (2016).
Valdes, P. et al. Control of dopaminergic neuron survival by the unfolded protein response transcription factor XBP1. Proc. Natl Acad. Sci. USA 111, 6804–6809 (2014).
Sado, M. et al. Protective effect against Parkinson's disease-related insults through the activation of XBP1. Brain Res. 1257, 16–24 (2009).
Zuleta, A., Vidal, R. L., Armentano, D., Parsons, G. & Hetz, C. AAV-mediated delivery of the transcription factor XBP1s into the striatum reduces mutant Huntingtin aggregation in a mouse model of Huntington's disease. Biochem. Biophys. Res. Commun. 420, 558–563 (2012).
Hyrskyluoto, A. et al. Ubiquitin-specific protease-14 reduces cellular aggregates and protects against mutant huntingtin-induced cell degeneration: involvement of the proteasome and ER stress-activated kinase IRE1α. Hum. Mol. Genet. 23, 5928–5939 (2014).
Hetz, C. et al. Unfolded protein response transcription factor XBP-1 does not influence prion replication or pathogenesis. Proc. Natl Acad. Sci. USA 105, 757–762 (2008).
Casas-Tinto, S. et al. The ER stress factor XBP1s prevents amyloid-β neurotoxicity. Hum. Mol. Genet. 20, 2144–2160 (2011).
Loewen, C. A. & Feany, M. B. The unfolded protein response protects from tau neurotoxicity in vivo. PLoS ONE 5, e13084 (2010).
Safra, M., Ben-Hamo, S., Kenyon, C. & Henis-Korenblit, S. The ire-1 ER stress-response pathway is required for normal secretory-protein metabolism in C. elegans. J. Cell Sci. 126, 4136–4146 (2013).
Morita, S. et al. Targeting ABL-IRE1α signaling spares ER-stressed pancreatic β cells to reverse autoimmune diabetes. Cell Metab. 25, 1207 (2017).
Mollereau, B. et al. Adaptive preconditioning in neurological diseases - therapeutic insights from proteostatic perturbations. Brain Res. 1648, 603–616 (2016).
Fouillet, A. et al. ER stress inhibits neuronal death by promoting autophagy. Autophagy 8, 915–926 (2012).
Egawa, N. et al. The endoplasmic reticulum stress sensor, ATF6α, protects against neurotoxin-induced dopaminergic neuronal death. J. Biol. Chem. 286, 7947–7957 (2011).
Hashida, K. et al. ATF6α promotes astroglial activation and neuronal survival in a chronic mouse model of Parkinson's disease. PLoS ONE 7, e47950 (2012).
Naranjo, J. R. et al. Activating transcription factor 6 derepression mediates neuroprotection in Huntington disease. J. Clin. Invest. 126, 627–638 (2016).
Fernandez-Fernandez, M. R., Ferrer, I. & Lucas, J. J. Impaired ATF6α processing, decreased Rheb and neuronal cell cycle re-entry in Huntington's disease. Neurobiol. Dis. 41, 23–32 (2011).
Yamamoto, K. et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6α and XBP1. Dev. Cell 13, 365–376 (2007).
Zhao, L., Longo-Guess, C., Harris, B. S., Lee, J. W. & Ackerman, S. L. Protein accumulation and neurodegeneration in the woozy mutant mouse is caused by disruption of SIL1, a cochaperone of BiP. Nat. Genet. 37, 974–979 (2005).
Filezac de L'Etang, A. et al. Marinesco-Sjogren syndrome protein SIL1 regulates motor neuron subtype-selective ER stress in ALS. Nat. Neurosci. 18, 227–238 (2015). This report identified the role of ER chaperones, such as SIL1, in determining vulnerability of motor neurons to ER stress in ALS.
Jin, H., Mimura, N., Kashio, M., Koseki, H. & Aoe, T. Late-onset of spinal neurodegeneration in knock-in mice expressing a mutant BiP. PLoS ONE 9, e112837 (2014).
Kakiuchi, C. et al. Functional polymorphisms of HSPA5: possible association with bipolar disorder. Biochem. Biophys. Res. Commun. 336, 1136–1143 (2005).
Kakiuchi, C. et al. Association analysis of HSP90B1 with bipolar disorder. J. Hum. Genet. 52, 794–803 (2007).
Kraus, A. et al. Calnexin deficiency leads to dysmyelination. J. Biol. Chem. 285, 18928–18938 (2010).
Denzel, A. et al. Early postnatal death and motor disorders in mice congenitally deficient in calnexin expression. Mol. Cell. Biol. 22, 7398–7404 (2002).
Bernard-Marissal, N., Sunyach, C., Marissal, T., Raoul, C. & Pettmann, B. Calreticulin levels determine onset of early muscle denervation by fast motoneurons of ALS model mice. Neurobiol. Dis. 73, 130–136 (2015).
Gonzalez-Perez, P. et al. Identification of rare protein disulfide isomerase gene variants in amyotrophic lateral sclerosis patients. Gene 566, 158–165 (2015).
Kwok, C. T. et al. Association studies indicate that protein disulfide isomerase is a risk factor in amyotrophic lateral sclerosis. Free Radic. Biol. Med. 58, 81–86 (2013).
Yang, Q. & Guo, Z. B. Polymorphisms in protein disulfide isomerase are associated with sporadic amyotrophic lateral sclerosis in the Chinese Han population. Int. J. Neurosci. 126, 607–611 (2016).
Woehlbier, U. et al. ALS-linked protein disulfide isomerase variants cause motor dysfunction. EMBO J. 35, 845–865 (2016). The first study to identify mutations in ER chaperones in ALS.
Jara, J. H. et al. Corticospinal motor neurons are susceptible to increased ER stress and display profound degeneration in the absence of UCHL1 function. Cereb. Cortex 25, 4259–4272 (2015).
Le, N. T. et al. Motor neuron disease, TDP-43 pathology, and memory deficits in mice expressing ALS-FTD-linked UBQLN2 mutations. Proc. Natl Acad. Sci. USA 113, E7580–E7589 (2016).
Castillo, V. et al. Functional role of the disulfide isomerase ERp57 in axonal regeneration. PLoS ONE 10, e0136620 (2015).
Gorbatyuk, M. S. et al. Glucose regulated protein 78 diminishes α-synuclein neurotoxicity in a rat model of Parkinson disease. Mol. Ther. 20, 1327–1337 (2012).
Gorbatyuk, M. S. et al. Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proc. Natl Acad. Sci. USA 107, 5961–5966 (2010).
Moreno, J. A. et al. Sustained translational repression by eIF2α-P mediates prion neurodegeneration. Nature 485, 507–511 (2012). This report showed that modulating eIF2α phosphorylation restores translation levels and reduces synaptic deficits in prion-related disease in mice.
Moreno, J. A. et al. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci. Transl. Med. 5, 206ra138 (2013).
Radford, H., Moreno, J. A., Verity, N., Halliday, M. & Mallucci, G. R. PERK inhibition prevents tau-mediated neurodegeneration in a mouse model of frontotemporal dementia. Acta Neuropathol. 130, 633–642 (2015).
Halliday, M. et al. Partial restoration of protein synthesis rates by the small molecule ISRIB prevents neurodegeneration without pancreatic toxicity. Cell Death Dis. 6, e1672 (2015).
Rojas-Rivera, D. et al. When PERK inhibitors turn out to be new potent RIPK1 inhibitors: critical issues on the specificity and use of GSK2606414 and GSK2656157. Cell Death Differ. 24, 1100–1110 (2017).
Halliday, M. et al. Repurposed drugs targeting eIF2α-P-mediated translational repression prevent neurodegeneration in mice. Brain 140, 1768–1783 (2017). This study identified and characterized the clinically-relevant drugs trazodone hydrochloride and dibenzoylmethane as potential neuroprotective drug targets that target proteostasis.
Stockwell, S. R. et al. Mechanism-based screen for G1/S checkpoint activators identifies a selective activator of EIF2AK3/PERK signalling. PLoS ONE 7, e28568 (2012).
Bruch, J. et al. PERK activation mitigates tau pathology in vitro and in vivo. EMBO Mol. Med. 9, 371–384 (2017).
Bruch, J. et al. Early neurodegeneration in the brain of a child without functional PKR-like endoplasmic reticulum kinase. J. Neuropathol. Exp. Neurol. 74, 850–857 (2015).
Yang, W. et al. Repression of the eIF2α kinase PERK alleviates mGluR-LTD impairments in a mouse model of Alzheimer's disease. Neurobiol. Aging 41, 19–24 (2016).
Ma, T. et al. Suppression of eIF2α kinases alleviates Alzheimer's disease-related plasticity and memory deficits. Nat. Neurosci. 16, 1299–1305 (2013). This study reported that abnormal PERK activation underlies AD-linked synaptic dysfunction and memory impairment.
Johnson, E. C. & Kang, J. A small molecule targeting protein translation does not rescue spatial learning and memory deficits in the hAPP-J20 mouse model of Alzheimer's disease. PeerJ 4, e2565 (2016).
Trinh, M. A. et al. Brain-specific disruption of the eIF2α kinase PERK decreases ATF4 expression and impairs behavioral flexibility. Cell Rep. 1, 676–688 (2012).
Trinh, M. A. et al. The eIF2α kinase PERK limits the expression of hippocampal metabotropic glutamate receptor-dependent long-term depression. Learn. Mem. 21, 298–304 (2014).
Buffington, S. A., Huang, W. & Costa-Mattioli, M. Translational control in synaptic plasticity and cognitive dysfunction. Annu. Rev. Neurosci. 37, 17–38 (2014).
Kakiuchi, C. et al. Impaired feedback regulation of XBP1 as a genetic risk factor for bipolar disorder. Nat. Genet. 35, 171–175 (2003).
Cheng, D., Zhang, K., Zhen, G. & Xue, Z. The -116C/G polymorphism in XBP1 gene is associated with psychiatric illness in Asian population: a meta-analysis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 165B, 665–672 (2014).
Liu, S. Y. et al. Polymorphism -116C/G of human X-box-binding protein 1 promoter is associated with risk of Alzheimer's disease. CNS Neurosci. Ther. 19, 229–234 (2013).
Martinez, G. et al. Regulation of memory formation by the transcription factor XBP1. Cell Rep. 14, 1382–1394 (2016). The first paper to describe a novel function of XBP1 in regulating learning and memory.
Cisse, M. et al. The transcription factor XBP1s restores hippocampal synaptic plasticity and memory by control of the Kalirin-7 pathway in Alzheimer model. Mol. Psychiatry http://dx.doi.org/10.1038/mp.2016.152 (2016).
Hayashi, A. et al. The role of brain-derived neurotrophic factor (BDNF)-induced XBP1 splicing during brain development. J. Biol. Chem. 282, 34525–34534 (2007).
Hayashi, A., Kasahara, T., Kametani, M. & Kato, T. Attenuated BDNF-induced upregulation of GABAergic markers in neurons lacking Xbp1. Biochem. Biophys. Res. Commun. 376, 758–763 (2008).
Clayton, B. L. & Popko, B. Endoplasmic reticulum stress and the unfolded protein response in disorders of myelinating glia. Brain Res. 1648, 594–602 (2016).
Lin, W. et al. The integrated stress response prevents demyelination by protecting oligodendrocytes against immune-mediated damage. J. Clin. Invest. 117, 448–456 (2007).
Lin, W. et al. Oligodendrocyte-specific activation of PERK signaling protects mice against experimental autoimmune encephalomyelitis. J. Neurosci. 33, 5980–5991 (2013).
Way, S. W. et al. Pharmaceutical integrated stress response enhancement protects oligodendrocytes and provides a potential multiple sclerosis therapeutic. Nat. Commun. 6, 6532 (2015).
Li, S., Yang, L., Selzer, M. E. & Hu, Y. Neuronal endoplasmic reticulum stress in axon injury and neurodegeneration. Ann. Neurol. 74, 768–777 (2013).
Valenzuela, V. et al. Activation of the unfolded protein response enhances motor recovery after spinal cord injury. Cell Death Dis. 3, e272 (2012).
Ohri, S. S. et al. Attenuating the endoplasmic reticulum stress response improves functional recovery after spinal cord injury. Glia 59, 1489–1502 (2011).
Ohri, S. S., Hetman, M. & Whittemore, S. R. Restoring endoplasmic reticulum homeostasis improves functional recovery after spinal cord injury. Neurobiol. Dis. 58, 29–37 (2013).
Ohri, S. S., Mullins, A., Hetman, M. & Whittemore, S. R. Inhibition of GADD34, the stress-inducible regulatory subunit of the endoplasmic reticulum stress response, does not enhance functional recovery after spinal cord injury. PLoS ONE 9, e109703 (2014).
Larhammar, M. et al. Dual leucine zipper kinase-dependent PERK activation contributes to neuronal degeneration following insult. eLife 6, e20725 (2017).
Saraswat Ohri, S., Hetman, M. & Whittemore, S. R. ATF6α deletion modulates the ER stress response after spinal cord injury but does not affect locomotor recovery. J. Neurotrauma http://dx.doi.org/10.1089/neu.2015.3993 (2016).
Qi, X., Hosoi, T., Okuma, Y., Kaneko, M. & Nomura, Y. Sodium 4-phenylbutyrate protects against cerebral ischemic injury. Mol. Pharmacol. 66, 899–908 (2004).
Mizukami, T. et al. Sodium 4-phenylbutyrate protects against spinal cord ischemia by inhibition of endoplasmic reticulum stress. J. Vasc. Surg. 52, 1580–1586 (2010). An important study that showed that PERK activation in oligodendrocytes reduces the severity of multiple sclerosis.
Onate, M. et al. Activation of the unfolded protein response promotes axonal regeneration after peripheral nerve injury. Sci. Rep. 6, 21709 (2016).
Mattson, M. P., Guo, Q., Furukawa, K. & Pedersen, W. A. Presenilins, the endoplasmic reticulum, and neuronal apoptosis in Alzheimer's disease. J. Neurochem. 70, 1–14 (1998).
Soto, C. Transmissible proteins: expanding the prion heresy. Cell 149, 968–977 (2012).
Ansar, M. et al. Mutation of ATF6 causes autosomal recessive achromatopsia. Hum. Genet. 134, 941–950 (2015).
Kohl, S. et al. Mutations in the unfolded protein response regulator ATF6 cause the cone dysfunction disorder achromatopsia. Nat. Genet. 47, 757–765 (2015).
Chiang, W. C. et al. Achromatopsia mutations target sequential steps of ATF6 activation. Proc. Natl Acad. Sci. USA 114, 400–405 (2016).
Godin, J. D., Creppe, C., Laguesse, S. & Nguyen, L. Emerging roles for the unfolded protein response in the developing nervous system. Trends Neurosci. 39, 394–404 (2016).
De Jaco, A. et al. A mutation linked with autism reveals a common mechanism of endoplasmic reticulum retention for the α, β-hydrolase fold protein family. J. Biol. Chem. 281, 9667–9676 (2006).
Ulbrich, L. et al. Autism-associated R451C mutation in neuroligin3 leads to activation of the unfolded protein response in a PC12 Tet-On inducible system. Biochem. J. 473, 423–434 (2016).
Obacz, J. et al. Endoplasmic reticulum proteostasis in glioblastoma—From molecular mechanisms to therapeutic perspectives. Sci. Signal. 10(2017).
Taylor, R. C. & Dillin, A. XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell 153, 1435–1447 (2013).
Williams, K. W. et al. Xbp1s in Pomc neurons connects ER stress with energy balance and glucose homeostasis. Cell Metab. 20, 471–482 (2014).
Acknowledgements
C.H. is supported by the FONDAP program 15150012, the US Office of Naval Research-Global (ONR-G) N62909-16-1-2003, the Millennium Institute P09-015-F, FONDEF ID16I10223, FONDEF D11E1007, the US Air Force Office of Scientific Research FA9550-16-1-0384, CONICYT-Brazil 441921/2016-7, the ALS Therapy Alliance 2014-F-059, the Muscular Dystrophy Association 382453, the Michael J Fox Foundation for Parkinson's Research – Target Validation grant No 9277, FONDECYT no. 1140549, and the ALSRP Therapeutic Idea Award AL150111. S.S. is supported by the Synapsis Foundation, Stiftung UNISCIENTIA, the Frick foundation for ALS research, Swiss National Science Foundation and European Research Council 725825.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1
Functional impact of ER stress on neuroinflammation and axonal damage. (PDF 107 kb)
Glossary
- Autophagy
-
Self-degradation process with functions that include the removal of misfolded or aggregated proteins and damaged organelles.
- Proteostasis
-
A portmanteau of the words protein and homeostasis, referring to the function of integrated biological pathways within cells that control the biogenesis, folding, trafficking and degradation of proteins present within and outside the cell.
- ER stress
-
A cellular condition that involves accumulation of misfolded and/or unfolded proteins at the ER; ER stress activates the unfolded protein response, which enables adaptation to stress or triggers apoptosis of irreversibly-damaged cells.
- Unfolded protein response
-
A signal transduction pathway that is activated by an accumulation of unfolded or misfolded proteins in the ER lumen; the unfolded protein response mediates adaptation to protein folding stress or the elimination of non-functional cells by apoptosis.
- ER-associated degradation
-
Cellular pathway that targets misfolded proteins at the ER for ubiquitylation and subsequent degradation in the cytosol by the proteasome.
- Integrated stress response
-
An adaptive pathway in eukaryotic cells that is activated by a range of stress conditions that converge on phosphorylation of eukaryotic translation initiation factor 2α, which leads to a decrease in global protein synthesis and the upregulation of selected genes that promote cellular homeostasis.
- Protein disulfide isomerase
-
One of a family of enzymes in the ER that catalyse the formation, isomerization and breakage of disulfide bonds between cysteine residues within proteins as they fold, enabling the correct arrangement of disulfide bonds in the fully folded state to form quickly.
- Hormesis
-
A phenomenon in which an agent that is toxic to a biological system at high doses has beneficial effects on that system at lower doses.
Rights and permissions
About this article
Cite this article
Hetz, C., Saxena, S. ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol 13, 477–491 (2017). https://doi.org/10.1038/nrneurol.2017.99
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2017.99
This article is cited by
-
Endoplasmic reticulum stress and the unfolded protein response: emerging regulators in progression of traumatic brain injury
Cell Death & Disease (2024)
-
A Ketogenic Diet may Improve Cognitive Function in Rats with Temporal Lobe Epilepsy by Regulating Endoplasmic Reticulum Stress and Synaptic Plasticity
Molecular Neurobiology (2024)
-
Orchestrierung der Entzündung bei Kontaktallergie durch angeborene Immun- und zelluläre Stressantworten
Allergo Journal (2024)
-
Dynamic changes in endoplasmic reticulum morphology and its contact with the plasma membrane in motor neurons in response to nerve injury
Cell and Tissue Research (2024)
-
SAM68 directs STING signaling to apoptosis in macrophages
Communications Biology (2024)