Abstract
Currently, five anti-TNF biologic agents are approved for the treatment of rheumatoid arthritis (RA): adalimumab, infliximab, etanercept, golimumab and certolizumab pegol. Formation of anti-drug antibodies (ADA) has been associated with all five agents. In the case of adalimumab and infliximab, immunogenicity is strongly linked to subtherapeutic serum drug levels and a lack of clinical response, but for the other three agents, data on immunogenicity are scarce, suggesting that further research would be valuable. Low ADA levels might not influence the efficacy of anti-TNF therapy, whereas high ADA levels impair treatment efficacy by considerably reducing unbound drug levels. Immunogenicity is not only an issue in patients treated with anti-TNF biologic agents; the immunogenicity of other therapeutic proteins, such as factor VIII and interferons, is well known and has been investigated for many years. The results of such studies suggest that investigations to determine the optimal treatment regimen (drug dosing, treatment schedule and co-medication) required to minimize the likelihood of ADA formation might be an effective and practical way to deal with the immunogenicity of anti-TNF biologic agents for RA.
Key Points
-
The anti-TNF agents currently approved for the treatment of rheumatoid arthritis are associated with the formation of anti-drug antibodies (ADA)
-
A substantial proportion of patients treated with adalimumab or infliximab develop detectable ADA, typically in the first 6 months of therapy
-
Low ADA levels do not impair the efficacy of anti-TNF therapy, since adequate drug levels remain, but high ADA levels impair treatment efficacy by considerably reducing unbound drug levels
-
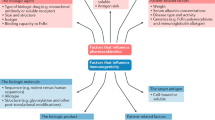
Many factors influence the immunogenicity of anti-TNF biologic agents: the drug's characteristics; the patient's genotype and immune system activity; dose, duration, administration route and co-treatment with immunomodulatory agents
-
Insight into how and why anti-TNF therapeutic antibodies evoke an immune response could lead to the development of strategies to minimize the adverse effects of existing anti-TNF agents
-
Study of immune responses in patients receiving anti-TNF agents could lead to techniques for preclinical identification and elimination of problematic epitopes during development of new drugs
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Elliott, M. J. et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor α (cA2) versus placebo in rheumatoid arthritis. Lancet 344, 1105–1110 (1994).
Weinblatt, M. E. et al. Adalimumab, a fully human anti-tumor necrosis factor α monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 48, 35–45 (2003).
Moreland, L. W. et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)–Fc fusion protein. N. Engl. J. Med. 337, 141–147 (1997).
Zhou, H. et al. Pharmacokinetics and safety of golimumab, a fully human anti-TNF-α monoclonal antibody, in subjects with rheumatoid arthritis. J. Clin. Pharmacol. 47, 383–396 (2007).
Choy, E. H. et al. Efficacy of a novel PEGylated humanized anti-TNF fragment (CDP870) in patients with rheumatoid arthritis: a phase II double-blinded, randomized, dose-escalating trial. Rheumatology (Oxford) 41, 1133–1137 (2002).
Chinol, M. et al. Biochemical modifications of avidin improve pharmacokinetics and biodistribution, and reduce immunogenicity. Br. J. Cancer 78, 189–197 (1998).
Baert, F. et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N. Engl. J. Med. 348, 601–608 (2003).
Pascual-Salcedo, D. et al. Influence of immunogenicity on the efficacy of long-term treatment with infliximab in rheumatoid arthritis. Rheumatology (Oxford) 50, 1445–1452 (2011).
Wolbink, G. J. et al. Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum. 54, 711–715 (2006).
Bendtzen, K. et al. Individualized monitoring of drug bioavailability and immunogenicity in rheumatoid arthritis patients treated with the tumor necrosis factor α inhibitor infliximab. Arthritis Rheum. 54, 3782–3789 (2006).
Svenson, M., Geborek, P., Saxne, T. & Bendtzen, K. Monitoring patients treated with anti-TNF-α biopharmaceuticals: assessing serum infliximab and anti-infliximab antibodies. Rheumatology (Oxford) 46, 1828–1834 (2007).
Jamnitski, A. et al. The presence or absence of antibodies to infliximab or adalimumab determines the outcome of switching to etanercept. Ann. Rheum. Dis. 70, 284–288 (2011).
van De Putte, L. B. et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann. Rheum. Dis. 63, 508–516 (2004).
Miyasaka, N. Clinical investigation in highly disease-affected rheumatoid arthritis patients in Japan with adalimumab applying standard and general evaluation: the CHANGE study. Mod. Rheumatol. 18, 252–262 (2008).
Rathanaswami, P. et al. Demonstration of an in vivo generated sub-picomolar affinity fully human monoclonal antibody to interleukin-8. Biochem. Biophys. Res. Commun. 334, 1004–1013 (2005).
Bray, G. L. et al. A multicenter study of recombinant factor VIII (recombinate): safety, efficacy, and inhibitor risk in previously untreated patients with hemophilia A. The Recombinate Study Group. Blood 83, 2428–2435 (1994).
Lusher, J. M., Arkin, S., Abildgaard, C. F. & Schwartz, R. S. Recombinant factor VIII for the treatment of previously untreated patients with hemophilia A. Safety, efficacy, and development of inhibitors. Kogenate Previously Untreated Patient Study Group. N. Engl. J. Med. 328, 453–459 (1993).
Antonelli, G. et al. Antibodies to interferon (IFN) in hepatitis C patients relapsing while continuing recombinant IFN-α2 therapy. Clin. Exp. Immunol. 104, 384–387 (1996).
Antonelli, G. Neutralising antibodies against interferon β in multiple sclerosis. Lancet 363, 168–169 (2004).
Giannelli, G. et al. Biological and clinical significance of neutralizing and binding antibodies to interferon-α (IFN-α) during therapy for chronic hepatitis C. Clin. Exp. Immunol. 97, 4–9 (1994).
Ross, C. et al. Immunogenicity of interferon-β in multiple sclerosis patients: influence of preparation, dosage, dose frequency, and route of administration. Danish Multiple Sclerosis Study Group. Ann. Neurol. 48, 706–712 (2000).
Weinblatt, M. E. et al. Adalimumab, a fully human anti-tumor necrosis factor α monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 48, 35–45 (2003).
Bartelds, G. M. et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA 305, 1460–1468 (2011).
Krieckaert, C., Rispens, T. & Wolbink, G. Immunogenicity of biological therapeutics: from assay to patient. Curr. Opin. Rheumatol. 24, 306–311 (2012).
Petitpain, N. et al. Arterial and venous thromboembolic events during anti-TNF therapy: a study of 85 spontaneous reports in the period 2000–2006. Biomed. Mater. Eng. 19, 355–364 (2009).
Makol, A., Grover, M., Guggenheim, C. & Hassouna, H. Etanercept and venous thromboembolism: a case series. J. Med. Case Rep. 4, 12 (2010).
Korswagen, L. A. et al. Venous and arterial thromboembolic events in adalimumab-treated patients with antiadalimumab antibodies: a case series and cohort study. Arthritis Rheum. 63, 877–883 (2011).
Dore, R. K. et al. The immunogenicity, safety, and efficacy of etanercept liquid administered once weekly in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 25, 40–46 (2007).
Jamnitski, A. et al. Patients non-responding to etanercept obtain lower etanercept concentrations compared with responding patients. Ann. Rheum. Dis. 71, 88–91 (2012).
Anderson, P. J. Tumor necrosis factor inhibitors: clinical implications of their different immunogenicity profiles. Semin. Arthritis Rheum. 34, 19–22 (2005).
Jacobi, A. M. et al. Activated memory B cell subsets correlate with disease activity in systemic lupus erythematosus: delineation by expression of CD27, IgD, and CD95. Arthritis Rheum. 58, 1762–1773 (2008).
Fleischmann, R. et al. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study. Ann. Rheum. Dis. 68, 805–811 (2009).
Smolen, J. et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann. Rheum. Dis. 68, 797–804 (2009).
Vennegoor, A. et al. Clinical relevance of serum natalizumab concentration and anti-natalizumab antibodies in multiple sclerosis. Mult. Scler. http://dx.doi.org/10.1177/1352458512460604.
Aarden, L., Ruuls, S. R. & Wolbink, G. Immunogenicity of anti-tumor necrosis factor antibodies—toward improved methods of anti-antibody measurement. Curr. Opin. Immunol. 20, 431–435 (2008).
Chirmule, N., Jawa, V. & Meibohm, B. Immunogenicity to therapeutic proteins: impact on PK/PD and efficacy. AAPS J. 14, 296–302 (2012).
Koren, E. et al. Recommendations on risk-based strategies for detection and characterization of antibodies against biotechnology products. J. Immunol. Methods 333, 1–9 (2008).
Hart, M. H. et al. Differential effect of drug interference in immunogenicity assays. J. Immunol. Methods 372, 196–203 (2011).
Wang, Y. M. et al. A survey of applications of biological products for drug interference of immunogenicity assays. Pharm. Res. http://dx.doi.org/10.1007/s11095-012-0833–2.
van Schouwenburg, P. A. et al. A novel method for the detection of antibodies to adalimumab in the presence of drug reveals “hidden” immunogenicity in rheumatoid arthritis patients. J. Immunol. Methods 362, 82–88 (2010).
Kosmac, M. et al. Exploring the binding sites of anti-infliximab antibodies in pediatric patients with rheumatic diseases treated with infliximab. Pediatr. Res. 69, 243–248 (2011).
Candon, S. et al. Clinical and biological consequences of immunization to infliximab in pediatric Crohn's disease. Clin. Immunol. 118, 11–19 (2006).
Vultaggio, A. et al. Anti-infliximab IgE and non-IgE antibodies and induction of infusion-related severe anaphylactic reactions. Allergy 65, 657–661 (2010).
van Schouwenburg, P. A. et al. IgG4 production against adalimumab during long term treatment of RA patients. J. Clin. Immunol. 32, 1000–1006 (2012).
Aalberse, R. C., van der Gaag, R. & van, L. J. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J. Immunol. 130, 722–726 (1983).
Baker, M. P., Reynolds, H. M., Lumicisi, B. & Bryson, C. J. Immunogenicity of protein therapeutics: the key causes, consequences and challenges. Self Nonself 1, 314–322 (2010).
Ben-Horin, S. et al. The immunogenic part of infliximab is the F(ab')2, but measuring antibodies to the intact infliximab molecule is more clinically useful. Gut 60, 41–48 (2011).
van Schouwenburg, P. A. et al. Adalimumab elicits a restricted anti-idiotypic antibody response in autoimmune patients resulting in functional neutralisation. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2012-201445.
Lobo, E. D., Hansen, R. J. & Balthasar, J. P. Antibody pharmacokinetics and pharmacodynamics. J. Pharm. Sci. 93, 2645–2668 (2004).
Moreland, L. W. et al. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann. Intern. Med. 130, 478–486 (1999).
Weinblatt, M. E. et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N. Engl. J. Med. 340, 253–259 (1999).
Lacroix-Desmazes, S. et al. Pathophysiology of inhibitors to factor VIII in patients with haemophilia A. Haemophilia 8, 273–279 (2002).
Rojas, J. R. et al. Formation, distribution, and elimination of infliximab and anti-infliximab immune complexes in cynomolgus monkeys. J. Pharmacol. Exp. Ther. 313, 578–585 (2005).
van der Laken, C. J. et al. Imaging and serum analysis of immune complex formation of radiolabelled infliximab and anti-infliximab in responders and non-responders to therapy for rheumatoid arthritis. Ann. Rheum. Dis. 66, 253–256 (2007).
Korswagen, L. A. et al. Venous and arterial thromboembolic events in adalimumab-treated patients with antiadalimumab antibodies: a case series and cohort study. Arthritis Rheum. 63, 877–883 (2011).
Carter, P. Improving the efficacy of antibody-based cancer therapies. Nat. Rev. Cancer 1, 118–129 (2001).
Clark, M. Antibody humanization: a case of the 'Emperor's new clothes'? Immunol. Today 21, 397–402 (2000).
Schellekens, H. Immunogenicity of therapeutic proteins: clinical implications and future prospects. Clin. Ther. 24, 1720–1740 (2002).
Bartelds, G. M. et al. Surprising negative association between IgG1 allotype disparity and anti-adalimumab formation: a cohort study. Arthritis Res. Ther. 12, R221 (2010).
Bartelds, G. M. et al. Anti-adalimumab antibodies in rheumatoid arthritis patients are associated with interleukin-10 gene polymorphisms. Arthritis Rheum. 60, 2541–2542 (2009).
Astermark, J. Prevention and prediction of inhibitor risk. Haemophilia 18 (Suppl. 4), 38–42 (2012).
Fakharzadeh, S. S. & Kazazian, H. H. Jr. Correlation between factor VIII genotype and inhibitor development in hemophilia A. Semin. Thromb. Hemost. 26, 167–171 (2000).
Maini, R. N. et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor α monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 41, 1552–1563 (1998).
Oldenburg, J., Schwaab, R. & Brackmann, H. H. Induction of immune tolerance in haemophilia A inhibitor patients by the 'Bonn Protocol': predictive parameter for therapy duration and outcome. Vox Sang. 77 (Suppl. 1), 49–54 (1999).
Schellekens, H. Factors influencing the immunogenicity of therapeutic proteins. Nephrol. Dial. Transplant. 20 (Suppl. 6), vi3–vi9 (2005).
Porter, S. Human immune response to recombinant human proteins. J. Pharm. Sci. 90, 1–11 (2001).
Konrad, M. W., Childs, A. L., Merigan, T. C. & Borden, E. C. Assessment of the antigenic response in humans to a recombinant mutant interferon β. J. Clin. Immunol. 7, 365–375 (1987).
Genovese, M. C. et al. Subcutaneous abatacept versus intravenous abatacept: a phase IIIb noninferiority study in patients with an inadequate response to methotrexate. Arthritis Rheum. 63, 2854–2864 (2011).
Carpenter, J. et al. Meeting report on protein particles and immunogenicity of therapeutic proteins: filling in the gaps in risk evaluation and mitigation. Biologicals 38, 602–611 (2010).
Chirino, A. J. & Mire-Sluis, A. Characterizing biological products and assessing comparability following manufacturing changes. Nat. Biotechnol. 22, 1383–1391 (2004).
Hermeling, S. et al. Antibody response to aggregated human interferon α2b in wild-type and transgenic immune tolerant mice depends on type and level of aggregation. J. Pharm. Sci. 95, 1084–1096 (2006).
Somerfield, J. et al. A novel strategy to reduce the immunogenicity of biological therapies. J. Immunol. 185, 763–768 (2010).
Kohno, T., Tam, L. T., Stevens, S. R. & Louie, J. S. Binding characteristics of tumor necrosis factor receptor–Fc fusion proteins vs anti-tumor necrosis factor mAbs. J. Investig. Dermatol. Symp. Proc. 12, 5–8 (2007).
Kim, M. S. et al. Comparative analyses of complex formation and binding sites between human tumor necrosis factor-α and its three antagonists elucidate their different neutralizing mechanisms. J. Mol. Biol. 374, 1374–1388 (2007).
High, K., Meng, Y., Washabaugh, M. W. & Zhao, Q. Determination of picomolar equilibrium dissociation constants in solution by enzyme-linked immunosorbent assay with fluorescence detection. Anal. Biochem. 347, 159–161 (2005).
Richards, J. et al. Phase I evaluation of humanized OKT3: toxicity and immunomodulatory effects of hOKT3γ4. Cancer Res. 59, 2096–2101 (1999).
Silva, H. M. et al. Novel humanized anti-CD3 antibodies induce a predominantly immunoregulatory profile in human peripheral blood mononuclear cells. Immunol. Lett. 125, 129–136 (2009).
Jamnitski, A. et al. Patients non-responding to etanercept obtain lower etanercept concentrations compared with responding patients. Ann. Rheum. Dis. 71, 88–91 (2012).
Brusic, V., Bajic, V. B. & Petrovsky, N. Computational methods for prediction of T-cell epitopes—a framework for modelling, testing, and applications. Methods 34, 436–443 (2004).
Jones, T. D. et al. Identification and removal of a promiscuous CD4+ T cell epitope from the C1 domain of factor VIII. J. Thromb. Haemost. 3, 991–1000 (2005).
Yeung, V. P. et al. Elimination of an immunodominant CD4+ T cell epitope in human IFN-β does not result in an in vivo response directed at the subdominant epitope. J. Immunol. 172, 6658–6665 (2004).
Moise, L. et al. Effect of HLA DR epitope de-immunization of factor VIII in vitro and in vivo. Clin. Immunol. 142, 320–331 (2012).
van Haren, S. D. et al. Requirements for immune recognition and processing of factor VIII by antigen-presenting cells. Blood Rev. 26, 43–49 (2012).
Stickler, M. M. et al. The human G1m1 allotype associates with CD4+ T-cell responsiveness to a highly conserved IgG1 constant region peptide and confers an asparaginyl endopeptidase cleavage site. Genes Immun. 12, 213–221 (2011).
Schellekens, H. Immunogenicity of therapeutic proteins. Nephrol. Dial. Transplant. 18, 1257–1259 (2003).
Jaffers, G. J. et al. Monoclonal antibody therapy. Anti-idiotypic and non-anti-idiotypic antibodies to OKT3 arising despite intense immunosuppression. Transplantation 41, 572–578 (1986).
Chatenoud, L. et al. The human immune response to the OKT3 monoclonal antibody is oligoclonal. Science 232, 1406–1408 (1986).
Chatenoud, L. et al. Restriction of the human in vivo immune response against the mouse monoclonal antibody OKT3. J. Immunol. 137, 830–838 (1986).
Magdelaine-Beuzelin, C. et al. IgG1 heavy chain-coding gene polymorphism (G1m allotypes) and development of antibodies-to-infliximab. Pharmacogenet. Genomics 19, 383–387 (2009).
Christen, U., Thuerkauf, R., Stevens, R. & Lesslauer, W. Immune response to a recombinant human TNFR55–IgG1 fusion protein: auto-antibodies in rheumatoid arthritis (RA) and multiple sclerosis (MS) patients have neither neutralizing nor agonist activities. Hum. Immunol. 60, 774–790 (1999).
Fulcher, C. A. et al. Localization of human factor FVIII inhibitor epitopes to two polypeptide fragments. Proc. Natl Acad. Sci. USA 82, 7728–7732 (1985).
Gilles, J. G. & Saint-Remy, J. M. Healthy subjects produce both anti-factor VIII and specific anti-idiotypic antibodies. J. Clin. Invest. 94, 1496–1505 (1994).
Lollar, P. et al. Inhibition of human factor VIIIa by anti-A2 subunit antibodies. J. Clin. Invest. 93, 2497–2504 (1994).
Prescott, R. et al. The inhibitor antibody response is more complex in hemophilia A patients than in most nonhemophiliacs with factor VIII autoantibodies. Recombinate and Kogenate Study Groups. Blood 89, 3663–3671 (1997).
Scandella, D. et al. Epitope specificity and functional characterization of factor VIII inhibitors. Adv. Exp. Med. Biol. 386, 47–63 (1995).
Mitoma, H. et al. Infliximab induces potent anti-inflammatory responses by outside-to-inside signals through transmembrane TNF-α. Gastroenterology 128, 376–392 (2005).
Scandella, D. et al. Epitope mapping of human factor VIII inhibitor antibodies by deletion analysis of factor VIII fragments expressed in Escherichia coli. Proc. Natl Acad. Sci. USA 85, 6152–6156 (1988).
Shima, M. et al. Factor VIII polypeptide specificity of monoclonal anti-factor VIII antibodies. Br. J. Haematol. 70, 63–69 (1988).
Parker, E. T. et al. Reduction of the inhibitory antibody response to human factor VIII in hemophilia A mice by mutagenesis of the A2 domain B-cell epitope. Blood 104, 704–710 (2004).
Mayer, A. et al. Modifying an immunogenic epitope on a therapeutic protein: a step towards an improved system for antibody-directed enzyme prodrug therapy (ADEPT). Br. J. Cancer 90, 2402–2410 (2004).
Tsutsumi, Y. et al. Site-specific chemical modification with polyethylene glycol of recombinant immunotoxin anti-Tac(Fv)–PE38 (LMB-2) improves antitumor activity and reduces animal toxicity and immunogenicity. Proc. Natl Acad. Sci. USA 97, 8548–8553 (2000).
Rosenberg, A. S. Effects of protein aggregates: an immunologic perspective. AAPS J. 8, E501–E507 (2006).
Lavigne-Lissalde, G., Schved, J. F., Granier, C. & Villard, S. Anti-factor VIII antibodies: a 2005 update. Thromb. Haemost. 94, 760–769 (2005).
Rosendaal, F. R. et al. A sudden increase in factor VIII inhibitor development in multitransfused hemophilia A patients in The Netherlands. Dutch Hemophilia Study Group. Blood 81, 2180–2186 (1993).
Locatelli, F., Del, V. L. & Pozzoni, P. Pure red-cell aplasia “epidemic”—mystery completely revealed? Perit. Dial. Int. 27 (Suppl. 2), S303–S307 (2007).
Jefferis, R. Glycosylation of recombinant antibody therapeutics. Biotechnol. Prog. 21, 11–16 (2005).
Author information
Authors and Affiliations
Contributions
All authors provided substantial contributions to discussions of content, and to reviewing and editing the manuscript before submission. P A van Schouwenburg researched the data and wrote the article.
Corresponding author
Ethics declarations
Competing interests
G. J. Wolbink declares that he is a member of the speakers' bureau or has received honoraria from Abbott, Amgen, BMS, Pfizer and UCB, and that he has received grant or research support from Wyeth Pharmaceuticals. T. Rispens declares that he is a member of the speakers' bureau or has received honoraria from Abbott and Pfizer. P. A. van Schouwenburg declares no competing interests.
Rights and permissions
About this article
Cite this article
van Schouwenburg, P., Rispens, T. & Wolbink, G. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nat Rev Rheumatol 9, 164–172 (2013). https://doi.org/10.1038/nrrheum.2013.4
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2013.4
This article is cited by
-
Characterization of anti-drug antibody dynamics using a bivariate mixed hidden-markov model by nonlinear-mixed effects approach
Journal of Pharmacokinetics and Pharmacodynamics (2024)
-
Analysis of ocular fluid in patients with ranibizumab-recalcitrant neovascular age-related macular degeneration who have serum anti-ranibizumab antibodies
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)
-
Utility of in silico prediction of target suppression for antibodies against soluble targets: static versus dynamic models
European Journal of Clinical Pharmacology (2023)
-
HLA-DQA1*05 and upstream variants of PPARGC1B are associated with infliximab persistence in Japanese Crohn’s disease patients
The Pharmacogenomics Journal (2023)
-
Immunogenicity of subcutaneous TNF inhibitors and its clinical significance in real-life setting in patients with spondyloarthritis
Rheumatology International (2022)