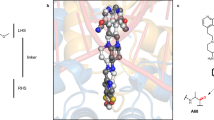
Abstract
Type II topoisomerases alter DNA topology by forming a covalent DNA-cleavage complex that allows DNA transport through a double-stranded DNA break. We present the structures of cleavage complexes formed by the Streptococcus pneumoniae ParC breakage-reunion and ParE TOPRIM domains of topoisomerase IV stabilized by moxifloxacin and clinafloxacin, two antipneumococcal fluoroquinolones. These structures reveal two drug molecules intercalated at the highly bent DNA gate and help explain antibacterial quinolone action and resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Schoeffler, A.J. & Berger, J.M. Q. Rev. Biophys. 41, 41–101 (2008).
Drlica, K. & Zhao, X. Microbiol. Mol. Biol. Rev. 61, 377–392 (1997).
Higgins, P.G., Fluit, A.C. & Schmitz, F.J. Curr. Drug Targets 4, 181–190 (2003).
Leo, E. et al. J. Biol. Chem. 280, 14252–14263 (2005).
Drlica, K., Malik, M., Kerns, R.J. & Zhao, X. Antimicrob. Agents Chemother. 52, 385–392 (2008).
Hooper, D.C. Lancet Infect. Dis. 2, 530–538 (2002).
Laponogov, I. et al. PLoS One 2, e301 (2007).
Corbett, K.D., Schoeffler, A.J., Thomsen, N.D. & Berger, J.M. J. Mol. Biol. 351, 545–561 (2005).
Morais Cabral, J.H. et al. Nature 388, 903–906 (1997).
Berger, J.M., Gamblin, S.J., Harrison, S.C. & Wang, J.C. Nature 379, 225–232 (1996).
McKay, D.B. & Steitz, T.A. Nature 290, 744–749 (1981).
Dong, K.C. & Berger, J.M. Nature 450, 1201–1205 (2007).
Rice, P.A., Yang, S., Mizuuchi, K. & Nash, H.A. Cell 87, 1295–1306 (1996).
Pan, X.-S. & Fisher, L.M. Antimicrob. Agents Chemother. 42, 2810–2816 (1998).
Pan, X.-S., Yague, G. & Fisher, L.M. Antimicrob. Agents Chemother. 45, 3140–3147 (2001).
Weigel, L.M., Anderson, G.J., Facklam, R.R. & Tenover, F.C. Antimicrob. Agents Chemother. 45, 3517–3523 (2001).
Yoshida, H., Bogaki, M., Nakamura, M., Yamanaka, L.M. & Nakamura, S. Antimicrob. Agents Chemother. 35, 1647–1650 (1991).
Shen, L.L. et al. Biochemistry 28, 3886–3894 (1989).
Kwok, Y., Zeng, Q. & Hurley, L.H. J. Biol. Chem. 274, 17226–17235 (1999).
Humphrey, W., Dalke, A. & Schulten, K. J. Mol. Graph. 14, 33–38 (1996).
Emsley, P. & Cowtan, K. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Acknowledgements
We thank the IO3 beamline personnel at the DIAMOND synchrotron for their help in collecting native and fixed Pt edge synchrotron data and P. Legrand for his help during MAD data collection at SOLEIL. J. Head and B. Seaton are thanked for providing us with OTCase X-ray diffraction data in space group P32 to test our search algorithms. G. Konrad of Metabion is thanked for her help regarding oligonucleotide synthesis and purification. I.L. was supported by St. George's, University of London. X.-S.P. and R.S. were supported by project grants BBD01882X1 and BBD0144841 (to L.M.F.) from the Biotechnology and Biological Sciences Research Council, UK, D.A.V. by the Guy's and St. Thomas Charitable Trust project grant R050701 and M.K.S. by the UK Medical Research Council.
Author information
Authors and Affiliations
Contributions
I.L. crystallized the present complexes, carried out data collection, HKL2000 data processing, structure determination and complete refinement, and wrote the paper; M.K.S. carried out data collection and earlier crystallization of other DNA–topo IV complexes; D.A.V. collected data; X.-S.P. and R.S. performed the biochemical experiments and overexpressed and purified the proteins for crystallization; A.W.T. and K.E.M. performed beamline direction and wavelength monochromation, synchrotron data collection and processing; L.M.F. conceived the experiments and wrote the paper; M.R.S. conceived the experiments, collected data, performed XDS data processing and refinement and wrote the paper.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3, Supplementary Table 1 and Supplementary Methods (PDF 528 kb)
Rights and permissions
About this article
Cite this article
Laponogov, I., Sohi, M., Veselkov, D. et al. Structural insight into the quinolone–DNA cleavage complex of type IIA topoisomerases. Nat Struct Mol Biol 16, 667–669 (2009). https://doi.org/10.1038/nsmb.1604
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1604
This article is cited by
-
Synthesis and in-vitro anti-proliferative with antimicrobial activity of new coumarin containing heterocycles hybrids
Scientific Reports (2023)
-
Evybactin is a DNA gyrase inhibitor that selectively kills Mycobacterium tuberculosis
Nature Chemical Biology (2022)
-
Fluoroquinolone resistance contributing mechanisms and genotypes of ciprofloxacin- unsusceptible Pseudomonas aeruginosa strains in Iran: emergence of isolates carrying qnr/aac(6)-Ib genes
International Microbiology (2022)
-
GyrB inhibitors as potential antibacterial agents: a review
Monatshefte für Chemie - Chemical Monthly (2021)
-
Microwave-assisted synthesis, molecular docking and anti-HIV activities of some drug-like quinolone derivatives
Medicinal Chemistry Research (2020)