Abstract
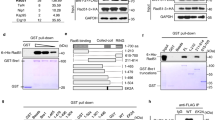
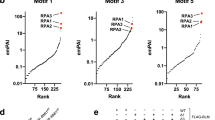
BRCA2 is a tumor suppressor that functions in homologous recombination, a key genomic integrity pathway. BRCA2 interacts with RAD51, the central protein of recombination, which forms filaments on single-stranded DNA (ssDNA) to perform homology search and DNA strand invasion. We report the purification of full-length human BRCA2 and show that it binds to ∼6 RAD51 molecules and promotes RAD51 binding to ssDNA coated by replication protein A (RPA), in a manner that is stimulated by DSS1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wooster, R. et al. Nature 378, 789–792 (1995).
Moynahan, M.E., Pierce, A.J. & Jasin, M. Mol. Cell 7, 263–272 (2001).
Wong, A.K.C., Pero, R., Ormonde, P.A., Tavtigian, S.V. & Bartel, P.L. J. Biol. Chem. 272, 31941–31944 (1997).
Esashi, F. et al. Nature 434, 598–604 (2005).
Yuan, S.S.F. et al. Cancer Res. 59, 3547–3551 (1999).
San Filippo, J. et al. J. Biol. Chem. 281, 11649–11657 (2006).
Yang, H. et al. Science 297, 1837–1848 (2002).
Yang, H., Li, Q.B., Fan, J., Holloman, W.K. & Pavletich, N.P. Nature 433, 653–657 (2005).
Davies, A.A. et al. Mol. Cell 7, 273–282 (2001).
Esashi, F., Galkin, V.E., Yu, X., Egelman, E.H. & West, S.C. Nat. Struct. Mol. Biol. 14, 468–474 (2007).
Carreira, A. et al. Cell 136, 1032–1043 (2009).
Saeki, H. et al. Proc. Natl. Acad. Sci. USA 103, 8768–8773 (2006).
Kojic, M., Kostrub, C.F., Buchman, A.R. & Holloman, W.K. Mol. Cell 10, 683–691 (2002).
Jensen, R.B., Carreira, A. & Kowalczykowski, S.C. Nature advance online publication, doi:10.1038/nature09399 (22 August 2010).
Hilario, J., Amitani, I., Baskin, R.J. & Kowalczykowski, S.C. Proc. Natl. Acad. Sci. USA 106, 361–368 (2009).
van der Heijden, T. et al. Nucleic Acids Res. 35, 5646–5657 (2007).
Bugreev, D.V. & Mazin, A.V. Proc. Natl. Acad. Sci. USA 101, 9988–9993 (2004).
Marston, N.J. et al. Mol. Cell. Biol. 19, 4633–4642 (1999).
Kojic, M., Yang, H.J., Kostrub, C.F., Pavletich, N.P. & Holloman, W.K. Mol. Cell 12, 1043–1049 (2003).
Gudmundsdottir, K., Lord, C.J., Witt, E., Tutt, A.N.J. & Ashworth, A. EMBO Rep. 5, 989–993 (2004).
Acknowledgements
We thank M. Wold (University of Iowa) and P. Sung (Yale University) for antibodies and overexpression vectors, and S. Kowalczykowski for sharing unpublished results and for comments, as well as N. Hunter, K. Ehmsen, E. Schwartz, W. Wright, X.-P. Zhang, D. Meyer, J. Sneeden and C. Fasching for critical comments on the manuscript. This work was supported by grants from the Tobacco-Related Disease Research Program (17FT-0046), US National Institutes of Health (GM58015, CA92276), US Department of Defense (DAMD17-00-1-0187), Susan G. Komen Breast Cancer Foundation (BCTR0201259) and University of California Davis Cancer Center.
Author information
Authors and Affiliations
Contributions
J.L. designed, performed and analyzed all experiments and helped write the manuscript. T.D., J.L. and B.G. purified BRCA2 and DSS1. W.-D.H. conceived the project, designed experiments, contributed to data analysis and wrote the manuscript with J.L., with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 and Supplementary Methods (PDF 852 kb)
Rights and permissions
About this article
Cite this article
Liu, J., Doty, T., Gibson, B. et al. Human BRCA2 protein promotes RAD51 filament formation on RPA-covered single-stranded DNA. Nat Struct Mol Biol 17, 1260–1262 (2010). https://doi.org/10.1038/nsmb.1904
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1904
This article is cited by
-
Bre1/RNF20 promotes Rad51-mediated strand exchange and antagonizes the Srs2/FBH1 helicases
Nature Communications (2023)
-
Repair of DNA double-strand breaks in plant meiosis: role of eukaryotic RecA recombinases and their modulators
Plant Reproduction (2023)
-
Expression of human BRCA2 in Saccharomyces cerevisiae complements the loss of RAD52 in double-strand break repair
Current Genetics (2023)
-
POLθ-mediated end joining is restricted by RAD52 and BRCA2 until the onset of mitosis
Nature Cell Biology (2021)
-
A complex of BRCA2 and PP2A-B56 is required for DNA repair by homologous recombination
Nature Communications (2021)