Abstract
Peptide transporters of the PepT family have key roles in the transport of di- and tripeptides across membranes as well as in the absorption of orally administered drugs in the small intestine. We have determined structures of a PepT transporter from Shewanella oneidensis (PepTSo2) in complex with three different peptides. The peptides bind in a large cavity lined by residues that are highly conserved in human PepT1 and PepT2. The bound peptides adopt extended conformations with their N termini clamped into a conserved polar pocket. A positively charged patch allows differential interactions with the C-terminal carboxylates of di- and tripeptides. Here we identify three pockets for peptide side chain interactions, and our binding studies define differential roles of these pockets for the recognition of different subtypes of peptide side chains.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Reddy, V.S., Shlykov, M.A., Castillo, R., Sun, E.I. & Saier, M.H. Jr. The major facilitator superfamily (MFS) revisited. FEBS J. 279, 2022–2035 (2012).
Terada, T. & Inui, K. Recent advances in structural biology of peptide transporters. Curr. Top. Membr. 70, 257–274 (2012).
Guan, L. & Kaback, H.R. Lessons from lactose permease. Annu. Rev. Biophys. Biomol. Struct. 35, 67–91 (2006).
Radestock, S. & Forrest, L.R. The alternating-access mechanism of MFS transporters arises from inverted-topology repeats. J. Mol. Biol. 407, 698–715 (2011).
Quistgaard, E.M., Low, C., Moberg, P., Tresaugues, L. & Nordlund, P. Structural basis for substrate transport in the GLUT-homology family of monosaccharide transporters. Nat. Struct. Mol. Biol. 20, 766–768 (2013).
Saier, M.H. Jr. & Paulsen, I.T. Whole genome analyses of transporters in spirochetes: Borrelia burgdorferi and Treponema pallidum. J. Mol. Microbiol. Biotechnol. 2, 393–399 (2000).
Daniel, H. Molecular and integrative physiology of intestinal peptide transport. Annu. Rev. Physiol. 66, 361–384 (2004).
Hagting, A., Kunji, E.R., Leenhouts, K.J., Poolman, B. & Konings, W.N. The di- and tripeptide transport protein of Lactococcus lactis. A new type of bacterial peptide transporter. J. Biol. Chem. 269, 11391–11399 (1994).
Brandsch, M. Transport of drugs by proton-coupled peptide transporters: pearls and pitfalls. Expert Opin. Drug Metab. Toxicol. 5, 887–905 (2009).
Brandsch, M. Drug transport via the intestinal peptide transporter PepT1. Curr. Opin. Pharmacol. 13, 881–887 (2013).
Terada, T. & Inui, K. Peptide transporters: structure, function, regulation and application for drug delivery. Curr. Drug Metab. 5, 85–94 (2004).
Newstead, S. et al. Crystal structure of a prokaryotic homologue of the mammalian oligopeptide-proton symporters, PepT1 and PepT2. EMBO J. 30, 417–426 (2011).
Solcan, N. et al. Alternating access mechanism in the POT family of oligopeptide transporters. EMBO J. 31, 3411–3421 (2012).
Guettou, F. et al. Structural insights into substrate recognition in proton-dependent oligopeptide transporters. EMBO Rep. 14, 804–810 (2013).
Doki, S. et al. Structural basis for dynamic mechanism of proton-coupled symport by the peptide transporter POT. Proc. Natl. Acad. Sci. USA 110, 11343–11348 (2013).
Löw, C. et al. High-throughput analytical gel filtration screening of integral membrane proteins for structural studies. Biochim. Biophys. Acta 1830, 3497–3508 (2013).
Ennifar, E., Carpentier, P., Ferrer, J.L., Walter, P. & Dumas, P. X-ray-induced debromination of nucleic acids at the Br K absorption edge and implications for MAD phasing. Acta Crystallogr. D Biol. Crystallogr. 58, 1262–1268 (2002).
Oliéric, V. et al. Using X-ray absorption spectra to monitor specific radiation damage to anomalously scattering atoms in macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 63, 759–768 (2007).
Fang, G., Konings, W.N. & Poolman, B. Kinetics and substrate specificity of membrane-reconstituted peptide transporter DtpT of Lactococcus lactis. J. Bacteriol. 182, 2530–2535 (2000).
Ito, K. et al. Analysing the substrate multispecificity of a proton-coupled oligopeptide transporter using a dipeptide library. Nat. Commun. 4, 2502 (2013).
Guettou, F. et al. Structural insights into substrate recognition in proton-dependent oligopeptide transporters. EMBO Rep. 14, 804–810 (2013).
Strong, M. et al. Toward the structural genomics of complexes: crystal structure of a PE/PPE protein complex from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 103, 8060–8065 (2006).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Chen, V.B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Jung, H., Tebbe, S., Schmid, R. & Jung, K. Unidirectional reconstitution and characterization of purified Na+/proline transporter of Escherichia coli. Biochemistry 37, 11083–11088 (1998).
Knol, J. et al. Unidirectional reconstitution into detergent-destabilized liposomes of the purified lactose transport system of Streptococcus thermophilus. J. Biol. Chem. 271, 15358–15366 (1996).
Peterson, G.L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 83, 346–356 (1977).
Acknowledgements
We thank the members of our group for suggestions and comments on the manuscript. This research was performed in P.N.'s research group and was supported by grants to P.N. from the Swedish Research council, the Swedish Cancer Society and the integrated EU project European drug initiative on channels and transporters (EDICT) as well as a Singapore National Research Foundation Competitive Research Program grant (NRF-CRP). We thank Diamond Light Source for access to beamline I03 (proposal mx6603) and BESSY for provision of synchrotron radiation facilities at beamline MX 14.3 in Berlin (proposal 2012_1_111073). We acknowledge the Protein Science Facility at the Karolinska Institutet for providing crystallization infrastructure. The research leading to these results has received further funding to P.N. from the European Community's Seventh Framework Programme (FP7/2007-2013) under BioStruct-X (grant agreement 783).
Author information
Authors and Affiliations
Contributions
C.L., F.G., M.R., P.N. and P.M. designed and performed experiments. C.L., E.M.Q., F.G., M.R. and P.N. wrote the manuscript. All authors discussed the results and commented on the manuscript. P.N. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Substrate coordination and Fo – Fc omit maps.
(A-C) PepTSo2 is shown as a cartoon representation with the N- and C-terminal halves colored in pink and blue respectively. The omit densities of the substrates are shown in green at a contour level of 3 σ. Important residues in the binding site are labeled and shown as sticks. The peptides AAA, AY(Br) and AY(Br)A are shown as sticks and colored green, magenta and blue respectively. Black dashes show potential hydrogen interaction between the substrate termini and the binding site residues. (D) PepTSo2 is presented as a grey colored cartoon. Important residues in the binding site are shown as blue sticks. One bromide anomalous density of the AY(Br)A substrate is presented as a green density contoured at 3 σ. The second bromide shows a strong negative Fo-Fc density countered here at 4 σ.
Supplementary Figure 2 Stereo view and samples of electron density maps for the AAA-bound protein.
(A) Stereo view of transmembrane helices 1, 5 and 6. The helices are labeled at the top of the panel and they are shown as sticks. 2Fo-Fc electron density map is contoured at 1.0 σ. (B) 2Fo-Fc electron density maps of helix 1 to 6 contoured at 1.0 σ, green density corresponds to the actual helix.
Supplementary Figure 3 Overall structure and conservation of PepTSo2 binding site.
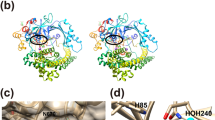
(A) Overview of the inward open conformation of PepTSo2 viewed from the plane of the membrane. The N- and C-terminal subdomains are colored pink and blue respectively. The protein is in complex with the tripeptide AY(Br)Y, shown as blue sticks with a black Br atom. The binding site residues are colored according to conservation. Red residues are identical while the yellow residues are similar to the human PepT homologues. Corresponding residues are presented in brackets. For creating the figure, alignment from 18 was used. (B) A close-up view of binding residues shown from the periplasmic side. The extended shape of the AY(Br)A peptide is visible, the N-terminus is coordinated by the conserved polar pocket (N151, E329 and E402). The C-terminus is coordinated by K121. The tyrosine side chain is clamped into the conserved hydrophobic pocket formed by F287, F288 and Y291.
Supplementary Figure 4 Superimposition of three peptide-bound structures including the antibacterial compound alafosfalin (4LEP)-bound structure.
The four known structures PepTSo2 are superimposed and shown in cartoon (protein) and sticks representations (peptide substrates). The structures are colored green (AAA), magenta (AY(Br)), blue (AY(Br)A) or yellow (alafosfalin). Average RMSD is 0.3 Å over 3000 atoms. There are minor variations in the C-terminal parts of H10 and H11.
Supplementary Figure 5 Wall-eyed stereo image of binding site and thermal aggregation assay versus uptake assay in proteoliposomes.
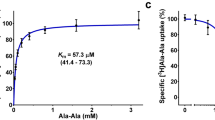
(A) The coordination of AY(Br)A is presented in a surface model where the binding site is divided into three pockets. Pocket 1 is colored in green, pocket 2 in yellow and pocket 3 in magenta. Important residues for substrate coordination are shown as sticks. (B) Peptide induced stabilization of PepTSo2. Thermal stability of PepTSo2 in presence of various peptides (5 mM) plotted as TΔ°C relative to the thermal stability of apo PepTSo2. The melting temperatures were derived from stargazer static light scattering unfolding curves. (C) Inhibition of [3H]-L-AlaAla uptake in the presence of various tripeptides. Error bars represent the standard deviation of triplicate measurements.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1 and 2 (PDF 2681 kb)
Rights and permissions
About this article
Cite this article
Guettou, F., Quistgaard, E., Raba, M. et al. Selectivity mechanism of a bacterial homolog of the human drug-peptide transporters PepT1 and PepT2. Nat Struct Mol Biol 21, 728–731 (2014). https://doi.org/10.1038/nsmb.2860
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2860
This article is cited by
-
Peptide transporter structure reveals binding and action mechanism of a potent PEPT1 and PEPT2 inhibitor
Communications Chemistry (2022)
-
Engineering and functional characterization of a proton-driven β-lactam antibiotic translocation module for bionanotechnological applications
Scientific Reports (2021)
-
Passive Internalization of Bioactive β-Casein Peptides into Phospholipid (POPC) Bilayers. Free Energy Landscapes from Unbiased Equilibrium MD Simulations at μs-Time Scale
Food Biophysics (2021)
-
Cellular thermal shift assay for the identification of drug–target interactions in the Plasmodium falciparum proteome
Nature Protocols (2020)
-
Transcriptional regulator ArcA mediates expression of oligopeptide transport systems both directly and indirectly in Shewanella oneidensis
Scientific Reports (2019)