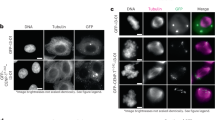
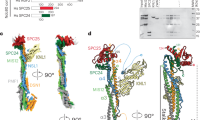
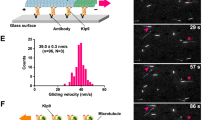
Abstract
The Dam1 kinetochore complex is essential for chromosome segregation in budding yeast. This ten-protein complex self-assembles around microtubules, forming ring-like structures that move with depolymerizing microtubule ends, a mechanism with implications for cellular function. Here we used EM-based single-particle and helical analyses to define the architecture of the Dam1 complex at 30-Å resolution and the self-assembly mechanism. Ring oligomerization seems to be facilitated by a conformational change upon binding to microtubules, suggesting that the Dam1 ring is not preformed, but self-assembles around kinetochore microtubules. The C terminus of the Dam1p protein, where most of the Aurora kinase Ipl1 phosphorylation sites reside, is in a strategic location to affect oligomerization and interactions with the microtubule. One of Ipl1's roles might be to fine-tune the coupling of the microtubule interaction with the conformational change required for oligomerization, with phosphorylation resulting in ring breakdown.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Cheeseman, I.M., Drubin, D.G. & Barnes, G. Simple centromere, complex kinetochore: linking spindle microtubules and centromeric DNA in budding yeast. J. Cell Biol. 157, 199–203 (2002).
McAinsh, A.D., Tytell, J.D. & Sorger, P.K. Structure, function, and regulation of budding yeast kinetochores. Annu. Rev. Cell Dev. Biol. 19, 519–539 (2003).
Maiato, H., DeLuca, J., Salmon, E.D. & Earnshaw, W.C. The dynamic kinetochore-microtubule interface. J. Cell Sci. 117, 5461–5477 (2004).
Hofmann, C. et al. Saccharomyces cerevisiae Duo1p and Dam1p, novel proteins involved in mitotic spindle function. J. Cell Biol. 143, 1029–1040 (1998).
Jones, M.H., Bachant, J.B., Castillo, A.R., Giddings, T.H., Jr. & Winey, M. Yeast Dam1p is required to maintain spindle integrity during mitosis and interacts with the Mps1p kinase. Mol. Biol. Cell 10, 2377–2391 (1999).
Cheeseman, I.M. et al. Implication of a novel multiprotein Dam1p complex in outer kinetochore function. J. Cell Biol. 155, 1137–1145 (2001).
Joglekar, A.P., Bouck, D.C., Molk, J.N., Bloom, K.S. & Salmon, E.D. Molecular architecture of a kinetochore-microtubule attachment site. Nat. Cell Biol. 8, 581–585 (2006).
Cheeseman, I.M. et al. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell 111, 163–172 (2002).
Shimogawa, M.M. et al. Mps1 phosphorylation of Dam1 couples kinetochores to microtubule plus ends at metaphase. Curr. Biol. 16, 1489–1501 (2006).
Westermann, S. et al. Formation of a dynamic kinetochore- microtubule interface through assembly of the Dam1 ring complex. Mol. Cell 17, 277–290 (2005).
Miranda, J.J., De Wulf, P., Sorger, P.K. & Harrison, S.C. The yeast DASH complex forms closed rings on microtubules. Nat. Struct. Mol. Biol. 12, 138–143 (2005).
Westermann, S. et al. The Dam1 kinetochore ring complex moves processively on depolymerizing microtubule ends. Nature 440, 565–569 (2006).
Nogales, E. & Wang, H.W. Structural intermediates in microtubule assembly and disassembly: how and why? Curr. Opin. Cell Biol. 18, 179–184 (2006).
Cheeseman, I.M., Enquist-Newman, M., Muller-Reichert, T., Drubin, D.G. & Barnes, G. Mitotic spindle integrity and kinetochore function linked by the Duo1p/Dam1p complex. J. Cell Biol. 152, 197–212 (2001).
Miranda, J.J., King, D.S. & Harrison, S.C. Protein arms in the kinetochore-microtubule interface of the yeast DASH complex. Mol. Biol. Cell published online 25 April 2007 (doi:10.1091/mbc.E07-02-0135).
Molodtsov, M.I., Grishchuk, E.L., Efremov, A.K., McIntosh, J.R. & Ataullakhanov, F.I. Force production by depolymerizing microtubules: a theoretical study. Proc. Natl. Acad. Sci. USA 102, 4353–4358 (2005).
Al-Bassam, J., Ozer, R.S., Safer, D., Halpain, S. & Milligan, R.A. MAP2 and tau bind longitudinally along the outer ridges of microtubule protofilaments. J. Cell Biol. 157, 1187–1196 (2002).
Mizuno, N. et al. Dynein and kinesin share an overlapping microtubule-binding site. EMBO J. 23, 2459–2467 (2004).
Hirose, K., Akimaru, E., Akiba, T., Endow, S.A. & Amos, L.A. Large conformational changes in a kinesin motor catalyzed by interaction with microtubules. Mol. Cell 23, 913–923 (2006).
Marx, A., Muller, J., Mandelkow, E.M., Hoenger, A. & Mandelkow, E. Interaction of kinesin motors, microtubules, and MAPs. J. Muscle Res. Cell Motil. 27, 125–137 (2006).
DeRosier, D., Stokes, D.L. & Darst, S.A. Averaging data derived from images of helical structures with different symmetries. J. Mol. Biol. 289, 159–165 (1999).
Tanaka, K. et al. Molecular mechanisms of kinetochore capture by spindle microtubules. Nature 434, 987–994 (2005).
Tan, D., Asenjo, A.B., Mennella, V., Sharp, D.J. & Sosa, H. Kinesin-13s form rings around microtubules. J. Cell Biol. 175, 25–31 (2006).
Moores, C.A. et al. The role of the kinesin-13 neck in microtubule depolymerization. Cell Cycle 5, 1812–1815 (2006).
Cheeseman, I.M., Chappie, J.S., Wilson-Kubalek, E.M. & Desai, A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127, 983–997 (2006).
Wei, R.R., Al-Bassam, J. & Harrison, S.C. The Ndc80/HEC1 complex is a contact point for kinetochore-microtubule attachment. Nat. Struct. Mol. Biol. 14, 54–59 (2007).
Cheng, Y. et al. Single particle reconstructions of the transferrin-transferrin receptor complex obtained with different specimen preparation techniques. J. Mol. Biol. 355, 1048–1065 (2006).
Radermacher, M., Wagenknecht, T., Verschoor, A. & Frank, J. Three-dimensional reconstruction from a single-exposure, random conical tilt series applied to the 50S ribosomal subunit of Escherichia coli. J. Microsc. 146, 113–136 (1987).
Penczek, P.A., Grassucci, R.A. & Frank, J. The ribosome at improved resolution: new techniques for merging and orientation refinement in 3D cryo-electron microscopy of biological particles. Ultramicroscopy 53, 251–270 (1994).
Schroeter, J.P. & Bretaudiere, J.P. SUPRIM: easily modified image processing software. J. Struct. Biol. 116, 131–137 (1996).
Wang, H.W. & Nogales, E. An iterative Fourier-Bessel algorithm for reconstruction of helical structures with severe Bessel overlap. J. Struct. Biol. 149, 65–78 (2005).
Pettersen, E.F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Goddard, T.D., Huang, C.C. & Ferrin, T.E. Visualizing density maps with UCSF Chimera. J. Struct. Biol. 157, 281–287 (2007).
Acknowledgements
We thank D. DeRosier for invaluable help with helical reconstruction of the assembled Dam1 complex and comments on the manuscript, J. Kuriyan and M. Lamers for help in performing the light-scattering experiments, E. Zelenova for help in preparing the helical crystal of Dam1 with microtubules, and J. Fang for Dam1 complex purification. This work was supported by grants from the National Institute of General Medical Sciences of the US National Institutes of Health (G.B. and E.N), from the Office of Biological and Environmental Research of the US Department of Energy (E.N.) and from Philip Morris USA of Philip Morris International (D.G.D.); by a postdoctoral fellowship of the Deutsche Forschungsgemeinschaft (S.W.); and by a predoctoral training grant from the US National Institutes of Health (V.H.R.). E.N. is a Howard Hughes Medical Institute Investigator.
Author information
Authors and Affiliations
Contributions
H.-W.W., S.W., D.G.D., G.B. and E.N. designed the research. H.-W.W. performed the electron microscopy and the helical reconstruction of the helical Dam1-microtubule assemblies. H.-W.W. and J.P.I.W. determined the handedness of the helical assembly. V.H.R. and A.E.L. performed the single-particle analysis of free Dam1 complexes in solution. S.W. prepared and purified the wild-type and mutant complexes. Y.N. built the GFP-labeled Dam1 construct and J.P.I.W. purified it. D.G.D. and G.B. supervised the biochemical experiments. E.N. supervised the electron microscopy and data analysis. H.-W.W., V.H.R. and E.N. performed the model docking and prepared the manuscript. All authors contributed to scientific discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Table 1 and Supplementary Methods (PDF 1585 kb)
Supplementary Video 1
Two monomers fit back in the dimer reconstruction of wild-type Dam1. The colors are coded the same as in Figure 2b. (MOV 2335 kb)
Rights and permissions
About this article
Cite this article
Wang, HW., Ramey, V., Westermann, S. et al. Architecture of the Dam1 kinetochore ring complex and implications for microtubule-driven assembly and force-coupling mechanisms. Nat Struct Mol Biol 14, 721–726 (2007). https://doi.org/10.1038/nsmb1274
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1274
This article is cited by
-
Regulation of kinetochore configuration during mitosis
Current Genetics (2018)
-
Differentiating the roles of microtubule-associated proteins at meiotic kinetochores during chromosome segregation
Chromosoma (2016)
-
The molecular architecture of the Dam1 kinetochore complex is defined by cross-linking based structural modelling
Nature Communications (2015)
-
Structural basis for microtubule recognition by the human kinetochore Ska complex
Nature Communications (2014)
-
Kinetochores require oligomerization of Dam1 complex to maintain microtubule attachments against tension and promote biorientation
Nature Communications (2014)