Abstract
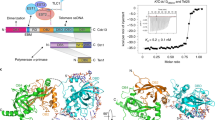
The POT1 (protection of telomeres 1) protein binds the single-stranded overhang at the ends of chromosomes in diverse eukaryotes. It is essential for chromosome end-protection in the fission yeast Schizosaccharomyces pombe, and it is involved in regulation of telomere length in human cells. Here, we report the crystal structure at a resolution of 1.73 Å of the N-terminal half of human POT1 (hPOT1) protein bound to a telomeric single-stranded DNA (ssDNA) decamer, TTAGGGTTAG, the minimum tight-binding sequence indicated by in vitro binding assays. The structure reveals that hPOT1 contains two oligonucleotide/ oligosaccharide-binding (OB) folds; the N-terminal OB fold binds the first six nucleotides, resembling the structure of the S. pombe Pot1pN–ssDNA complex, whereas the second OB fold binds and protects the 3′ end of the ssDNA. These results provide an atomic-resolution model for chromosome end-capping.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Blackburn, E.H. Switching and signaling at the telomere. Cell 106, 661–673 (2001).
Cervantes, R.B. & Lundblad, V. Mechanisms of chromosome-end protection. Curr. Opin. Cell Biol. 14, 351–356 (2002).
de Lange, T. Protection of mammalian telomeres. Oncogene 21, 532–540 (2002).
Cech, T.R. Beginning to understand the end of the chromosome. Cell 116, 273–279 (2004).
Bodnar, A.G. et al. Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352 (1998).
Rudolph, K.L. et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96, 701–712 (1999).
Hahn, W.C. et al. Inhibition of telomerase limits the growth of human cancer cells. Nat. Med. 5, 1164–1170 (1999).
Zhang, X., Mar, V., Zhou, W., Harrington, L. & Robinson, M.O. Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev. 13, 2388–2399 (1999).
Zakian, V.A. Telomeres: beginning to understand the end. Science 270, 1601–1607 (1995).
Wellinger, R.J., Wolf, A.J. & Zakian, V.A. Saccharomyces telomeres acquire single-strand TG1-3 tails late in S phase. Cell 72, 51–60 (1993).
Makarov, V.L., Hirose, Y. & Langmore, J.P. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell 88, 657–666 (1997).
Wright, W.E., Tesmer, V.M., Huffman, K.E., Levene, S.D. & Shay, J.W. Normal human chromosomes have long G-rich telomeric overhangs at one end. Genes Dev. 11, 2801–2809 (1997).
Baumann, P. & Cech, T.R. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292, 1171–1175 (2001).
Gray, J.T., Celander, D.W., Price, C.M. & Cech, T.R. Cloning and expression of genes for the Oxytricha telomere-binding protein: specific subunit interactions in the telomeric complex. Cell 67, 807–814 (1991).
Horvath, M.P., Schweiker, V.L., Bevilacqua, J.M., Ruggles, J.A. & Schultz, S.C. Crystal structure of the Oxytricha nova telomere end binding protein complexed with single strand DNA. Cell 95, 963–974 (1998).
Chandra, A., Hughes, T.R., Nugent, C.I. & Lundblad, V. Cdc13 both positively and negatively regulates telomere replication. Genes Dev. 15, 404–414 (2001).
Mitton-Fry, R.M., Anderson, E.M., Hughes, T.R., Lundblad, V. & Wuttke, D.S. Conserved structure for single-stranded telomeric DNA recognition. Science 296, 145–147 (2002).
Baumann, P., Podell, E. & Cech, T.R. Human Pot1 (protection of telomeres) protein: cytolocalization, gene structure, and alternative splicing. Mol. Cell. Biol. 22, 8079–8087 (2002).
Evans, S.K. & Lundblad, V. Positive and negative regulation of telomerase access to the telomere. J. Cell Sci. 113, 3357–3364 (2000).
Taggart, A.K., Teng, S.C. & Zakian, V.A. Est1p as a cell cycle-regulated activator of telomere-bound telomerase. Science 297, 1023–1026 (2002).
Garvik, B., Carson, M. & Hartwell, L. Single-stranded DNA arising at telomeres in cdc13 mutants may constitute a specific signal for the RAD9 checkpoint. Mol. Cell. Biol. 15, 6128–6138 (1995).
Lei, M., Baumann, P. & Cech, T.R. Cooperative binding of single-stranded telomeric DNA by the Pot1 protein of Schizosaccharomyces pombe. Biochemistry 41, 14560–14568 (2002).
Loayza, D. & de Lange, T. POT1 as a terminal transducer of TRF1 telomere length control. Nature 423, 1013–1018 (2003).
Colgin, L.M., Baran, K., Baumann, P., Cech, T.R. & Reddel, R.R. Human POT1 facilitates telomere elongation by telomerase. Curr. Biol. 13, 942–946 (2003).
Armbruster, B.N. et al. Rescue of an hTERT mutant defective in telomere elongation by fusion with hPot1. Mol. Cell. Biol. 24, 3552–3561 (2004).
Wei, C. & Price, C.M. Cell cycle localization, dimerization, and binding domain architecture of the telomere protein cPot1. Mol. Cell. Biol. 24, 2091–2102 (2004).
Loayza, D., Parsons, H., Donigian, J., Hoke, K. & de Lange, T. DNA binding features of human POT1: a nonamer 5′-TAGGGTTAG-3′ minimal binding site, sequence specificity, and internal binding to multimeric sites. J. Biol. Chem. 279, 13241–13248 (2004).
Murzin, A.G. OB(oligonucleotide/oligosaccharide binding)-fold: common structural and functional solution for non-homologous sequences. EMBO J. 12, 861–867 (1993).
Theobald, D.L., Mitton-Fry, R.M. & Wuttke, D.S. Nucleic acid recognition by OB-fold proteins. Annu. Rev. Biophys. Biomol. Struct. 32, 115–133 (2003).
Bochkarev, A., Pfuetzner, R.A., Edwards, A.M. & Frappier, L. Structure of the single-stranded-DNA-binding domain of replication protein A bound to DNA. Nature 385, 176–181 (1997).
Raghunathan, S., Ricard, C.S., Lohman, T.M. & Waksman, G. Crystal structure of the homo-tetrameric DNA binding domain of Escherichia coli single-stranded DNA-binding protein determined by multiwavelength X-ray diffraction on the selenomethionyl protein at 2.9-A resolution. Proc. Natl. Acad. Sci. USA 94, 6652–6657 (1997).
Lei, M., Podell, E.R., Baumann, P. & Cech, T.R. DNA self-recognition in the structure of Pot1 bound to telomeric single-stranded DNA. Nature 426, 198–203 (2003).
Theobald, D.L. & Wuttke, D.S. Prediction of multiple tandem OB-fold domains in telomere end-binding proteins Pot1 and Cdc13. Structure 12, 1877–1879 (2004).
Mitton-Fry, R.M., Anderson, E.M., Theobald, D.L., Glustrom, L.W. & Wuttke, D.S. Structural basis for telomeric single-stranded DNA recognition by yeast Cdc13. J. Mol. Biol. 338, 241–255 (2004).
Yang, H. et al. BRCA2 function in DNA binding and recombination from a BRCA2-DSS1-ssDNA structure. Science 297, 1837–1848 (2002).
Morin, G.B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell 59, 521–529 (1989).
Blasco, M.A., Funk, W., Villeponteau, B. & Greider, C.W. Functional characterization and developmental regulation of mouse telomerase RNA. Science 269, 1267–1270 (1995).
Holm, L. & Sander, C. Database algorithm for generating protein backbone and side-chain co-ordinates from a Cα trace application to model building and detection of co-ordinate errors. J. Mol. Biol. 218, 183–194 (1991).
Ding, J. et al. Crystal structure of the two-RRM domain of hnRNP A1 (UP1) complexed with single-stranded telomeric DNA. Genes Dev. 13, 1102–1115 (1999).
Lingner, J. & Cech, T.R. Purification of telomerase from Euplotes aediculatus: requirement of a primer 3′ overhang. Proc. Natl. Acad. Sci. USA 93, 10712–10717 (1996).
Liu, D. et al. PTOP interacts with POT1 and regulates its localization to telomeres. Nat. Cell Biol. 6, 673–680 (2004).
Ye, J.Z. et al. POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes Dev. 18, 1649–1654 (2004).
Griffith, J.D. et al. Mammalian telomeres end in a large duplex loop. Cell 97, 503–514 (1999).
Nikitina, T. & Woodcock, C.L. Closed chromatin loops at the ends of chromosomes. J. Cell Biol. 166, 161–165 (2004).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Brunger, A.T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994).
Esnouf, R.M. Further additions to MolScript version 1.4, including reading and contouring of electron-density maps. Acta Crystallogr. D 55, 938–940 (1999).
Merritt, E.A. & Murphy, M.E.P. Raster 3D version 2.0, a program for photorealistic molecular graphics. Acta Crystallogr. D 50, 869–873 (1994).
Nicholls, A., Sharp, K.A. & Honig, B. A rapid finite difference algorithm, utilizing successive overrelaxation to solve Poisson Boltzmann equation. J. Comput. Chem. 270, 26184–26191 (1991).
Kraulis, P.J. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structure. J. Appl. Crystallogr. 24, 945–950 (1991).
Chen, J.L., Blasco, M.A. & Greider, C.W. Secondary structure of vertebrate telomerase RNA. Cell 100, 503–514 (2000).
Acknowledgements
We thank L. Chen and D. Theobald (University of Colorado-Boulder) for insightful discussions and C. Ralston and G. McDermett (Advanced Light Source) for help in data collection. We thank K. Christensen and the tissue culture core facility of the University of Colorado Cancer Center for virus amplification and protein expression. This work was supported in part by a grant from the US National Institutes of Health. M.L. is an Agouron/Paul Sigler research fellow of the Helen Hay Whitney Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Two versions of hPOT1 (V1 = full-length hPOT1, V2 = N-terminal DNA-binding region) have equivalent DNA-binding properties. (PDF 84 kb)
Supplementary Fig. 2
Adding a 5′ end nucleotide (C) rescues the ssDNA-binding activity of 9mer (TAGGGTTAG). (PDF 56 kb)
Supplementary Fig. 3
Extra 5′ end nucleotides do not interfere with the hPOT1-ssDNA interaction, whereas as extra 3′ end nucleotides weaken the interaction. (PDF 62 kb)
Supplementary Fig. 4
Protein-ssDNA interactions between G10 and its peripheral protein residues. (PDF 85 kb)
Supplementary Fig. 5
hPOT1 contains two OB folds. (PDF 429 kb)
Supplementary Fig. 6
Structure-based sequence alignment of the Pot1 proteins of several vertebrates (human, mouse, rat and chicken). (PDF 951 kb)
Rights and permissions
About this article
Cite this article
Lei, M., Podell, E. & Cech, T. Structure of human POT1 bound to telomeric single-stranded DNA provides a model for chromosome end-protection. Nat Struct Mol Biol 11, 1223–1229 (2004). https://doi.org/10.1038/nsmb867
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb867
This article is cited by
-
Dynamic interaction of BRCA2 with telomeric G-quadruplexes underlies telomere replication homeostasis
Nature Communications (2022)
-
Structure of active human telomerase with telomere shelterin protein TPP1
Nature (2022)
-
KAS-seq: genome-wide sequencing of single-stranded DNA by N3-kethoxal–assisted labeling
Nature Protocols (2022)
-
Shaping human telomeres: from shelterin and CST complexes to telomeric chromatin organization
Nature Reviews Molecular Cell Biology (2021)
-
Structures of telomerase at several steps of telomere repeat synthesis
Nature (2021)