Abstract
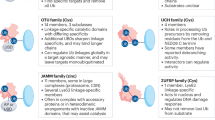
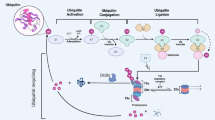
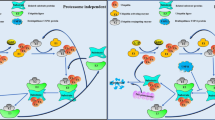
Deubiquitinases (DUBs) have fundamental roles in the ubiquitin system through their ability to specifically deconjugate ubiquitin from targeted proteins. The human genome encodes at least 98 DUBs, which can be grouped into 6 families, reflecting the need for specificity in their function. The activity of these enzymes affects the turnover rate, activation, recycling and localization of multiple proteins, which in turn is essential for cell homeostasis, protein stability and a wide range of signaling pathways. Consistent with this, altered DUB function has been related to several diseases, including cancer. Thus, multiple DUBs have been classified as oncogenes or tumor suppressors because of their regulatory functions on the activity of other proteins involved in tumor development. Therefore, recent studies have focused on pharmacological intervention on DUB activity as a rationale to search for novel anticancer drugs. This strategy may benefit from our current knowledge of the physiological regulatory mechanisms of these enzymes and the fact that growth of several tumors depends on the normal activity of certain DUBs. Further understanding of these processes may provide answers to multiple remaining questions on DUB functions and lead to the development of DUB-targeting strategies to expand the repertoire of molecular therapies against cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Adler AS, Littlepage LE, Lin M, Kawahara TL, Wong DJ, Werb Z et al. (2008). CSN5 isopeptidase activity links COP9 signalosome activation to breast cancer progression. Cancer Res 68: 506–515.
Al-Hakim A, Escribano-Diaz C, Landry MC, O'Donnell L, Panier S, Szilard RK et al. (2010). The ubiquitous role of ubiquitin in the DNA damage response. DNA Repair (Amst) 9: 1229–1240.
Al-Hakim AK, Zagorska A, Chapman L, Deak M, Peggie M, Alessi DR . (2008). Control of AMPK-related kinases by USP9X and atypical Lys(29)/Lys(33)-linked polyubiquitin chains. Biochem J 411: 249–260.
Alwan HA, van Leeuwen JE . (2007). UBPY-mediated epidermal growth factor receptor (EGFR) de-ubiquitination promotes EGFR degradation. J Biol Chem 282: 1658–1669.
Amerik AY, Hochstrasser M . (2004). Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta 1695: 189–207.
Aressy B, Jullien D, Cazales M, Marcellin M, Bugler B, Burlet-Schiltz O et al. (2010). A screen for deubiquitinating enzymes involved in the G/M checkpoint identifies USP50 as a regulator of HSP90-dependent Wee1 stability. Cell Cycle 9: 3815–3822.
Atanassov BS, Evrard YA, Multani AS, Zhang Z, Tora L, Devys D et al. (2009). Gcn5 and SAGA regulate shelterin protein turnover and telomere maintenance. Mol Cell 35: 352–364.
Atanassov BS, Koutelou E, Dent SY . (2011). The role of deubiquitinating enzymes in chromatin regulation. FEBS Lett 585: 2016–2023.
Baek KH . (2006). Cytokine-regulated protein degradation by the ubiquitination system. Curr Protein Pept Sci 7: 171–177.
Baek KH, Kim MS, Kim YS, Shin JM, Choi HK . (2004). DUB-1A, a novel deubiquitinating enzyme subfamily member, is polyubiquitinated and cytokine-inducible in B-lymphocytes. J Biol Chem 279: 2368–2376.
Balakirev MY, Tcherniuk SO, Jaquinod M, Chroboczek J . (2003). Otubains: a new family of cysteine proteases in the ubiquitin pathway. EMBO Rep 4: 517–522.
Bedford L, Lowe J, Dick LR, Mayer RJ, Brownell JE . (2011). Ubiquitin-like protein conjugation and the ubiquitin-proteasome system as drug targets. Nat Rev Drug Discov 10: 29–46.
Bott M, Brevet M, Taylor BS, Shimizu S, Ito T, Wang L et al. (2011). The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet 43: 668–672.
Bremm A, Freund SM, Komander D . (2010). Lys11-linked ubiquitin chains adopt compact conformations and are preferentially hydrolyzed by the deubiquitinase Cezanne. Nat Struct Mol Biol 17: 939–947.
Brooks CL, Li M, Hu M, Shi Y, Gu W . (2007). The p53—Mdm2—HAUSP complex is involved in p53 stabilization by HAUSP. Oncogene 26: 7262–7266.
Brummelkamp TR, Nijman SM, Dirac AM, Bernards R . (2003). Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature 424: 797–801.
Burnett B, Li F, Pittman RN . (2003). The polyglutamine neurodegenerative protein ataxin-3 binds polyubiquitylated proteins and has ubiquitin protease activity. Hum Mol Genet 12: 3195–3205.
Burrows JF, McGrattan MJ, Rascle A, Humbert M, Baek KH, Johnston JA . (2004). DUB-3, a cytokine-inducible deubiquitinating enzyme that blocks proliferation. J Biol Chem 279: 13993–14000.
Cao Z, Wu X, Yen L, Sweeney C, Carraway III KL . (2007). Neuregulin-induced ErbB3 downregulation is mediated by a protein stability cascade involving the E3 ubiquitin ligase Nrdp1. Mol Cell Biol 27: 2180–2188.
Clague MJ, Coulson JM, Urbe S . (2008). Deciphering histone 2A deubiquitination. Genome Biol 9: 202.
Clague MJ, Urbe S . (2006). Endocytosis: the DUB version. Trends Cell Biol 16: 551–559.
Colland F . (2010). The therapeutic potential of deubiquitinating enzyme inhibitors. Biochem Soc Trans 38: 137–143.
Colland F, Formstecher E, Jacq X, Reverdy C, Planquette C, Conrath S et al. (2009). Small-molecule inhibitor of USP7/HAUSP ubiquitin protease stabilizes and activates p53 in cells. Mol Cancer Ther 8: 2286–2295.
Colombo M, Vallese S, Peretto I, Jacq X, Rain JC, Colland F et al. (2010). Synthesis and biological evaluation of 9-oxo-9H-indeno[1,2-b]pyrazine-2,3-dicarbonitrile analogues as potential inhibitors of deubiquitinating enzymes. Chem Med Chem 5: 552–558.
Cooper EM, Cutcliffe C, Kristiansen TZ, Pandey A, Pickart CM, Cohen RE . (2009). K63-specific deubiquitination by two JAMM/MPN+ complexes: BRISC-associated Brcc36 and proteasomal Poh1. EMBO J 28: 621–631.
Coornaert B, Baens M, Heyninck K, Bekaert T, Haegman M, Staal J et al. (2008). T cell antigen receptor stimulation induces MALT1 paracaspase-mediated cleavage of the NF-kappaB inhibitor A20. Nat Immunol 9: 263–271.
Crawford LJ, Walker B, Irvine AE . (2011). Proteasome inhibitors in cancer therapy. J Cell Commun Signal 5: 101–110.
Chan DA, Giaccia AJ . (2011). Harnessing synthetic lethal interactions in anticancer drug discovery. Nat Rev Drug Discov 10: 351–364.
Chen M, Gutierrez GJ, Ronai ZA . (2011). Ubiquitin-recognition protein Ufd1 couples the endoplasmic reticulum (ER) stress response to cell cycle control. Proc Natl Acad Sci USA 108: 9119–9124.
Dammer EB, Na CH, Xu P, Seyfried NT, Duong DM, Cheng D et al. (2011). Polyubiquitin linkage profiles in three models of proteolytic stress suggest etiology of Alzheimer disease. J Biol Chem 286: 10457–10465.
Daviet L, Colland F . (2008). Targeting ubiquitin specific proteases for drug discovery. Biochimie 90: 270–283.
Dayal S, Sparks A, Jacob J, Allende-Vega N, Lane DP, Saville MK . (2009). Suppression of the deubiquitinating enzyme USP5 causes the accumulation of unanchored polyubiquitin and the activation of p53. J Biol Chem 284: 5030–5041.
de la Vega M, Kelvin AA, Dunican DJ, McFarlane C, Burrows JF, Jaworski J et al. (2011). The deubiquitinating enzyme USP17 is essential for GTPase subcellular localization and cell motility. Nat Commun 2: 259.
Drag M, Salvesen GS . (2010). Emerging principles in protease-based drug discovery. Nat Rev Drug Discov 9: 690–701.
Duex JE, Comeau L, Sorkin A, Purow B, Kefas B . (2011). USP18 regulates EGF receptor expression and cancer cell survival via microrna-7. J Biol Chem 286: 25377–25386.
Duex JE, Sorkin A . (2009). RNA interference screen identifies Usp18 as a regulator of epidermal growth factor receptor synthesis. Mol Biol Cell 20: 1833–1844.
Dupont S, Mamidi A, Cordenonsi M, Montagner M, Zacchigna L, Adorno M et al. (2009). FAM/USP9x, a deubiquitinating enzyme essential for TGFbeta signaling, controls Smad4 monoubiquitination. Cell 136: 123–135.
Durkop H, Hirsch B, Hahn C, Foss HD, Stein H . (2003). Differential expression and function of A20 and TRAF1 in Hodgkin lymphoma and anaplastic large cell lymphoma and their induction by CD30 stimulation. J Pathol 200: 229–239.
Eletr ZM, Wilkinson KD . (2011). An emerging model for BAP1's role in regulating cell cycle progression. Cell Biochem Biophys 60: 3–11.
Endo A, Matsumoto M, Inada T, Yamamoto A, Nakayama KI, Kitamura N et al. (2009). Nucleolar structure and function are regulated by the deubiquitylating enzyme USP36. J Cell Sci 122: 678–686.
Enesa K, Ito K, Luong le A, Thorbjornsen I, Phua C, To Y et al. (2008a). Hydrogen peroxide prolongs nuclear localization of NF-kappaB in activated cells by suppressing negative regulatory mechanisms. J Biol Chem 283: 18582–18590.
Enesa K, Zakkar M, Chaudhury H, Luong le A, Rawlinson L, Mason JC et al. (2008b). NF-kappaB suppression by the deubiquitinating enzyme Cezanne: a novel negative feedback loop in pro-inflammatory signaling. J Biol Chem 283: 7036–7045.
Fan YH, Yu Y, Mao RF, Tan XJ, Xu GF, Zhang H et al. (2011). USP4 targets TAK1 to downregulate TNFalpha-induced NF-kappaB activation. Cell Death Differ 18: 1547–1560.
Feng L, Wang J, Chen J . (2010). The Lys63-specific deubiquitinating enzyme BRCC36 is regulated by two scaffold proteins localizing in different subcellular compartments. J Biol Chem 285: 30982–30988.
Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D et al. (2011). COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res 39: D945–D950.
Frederick A, Rolfe M, Chiu MI . (1998). The human UNP locus at 3p21.31 encodes two tissue-selective, cytoplasmic isoforms with deubiquitinating activity that have reduced expression in small cell lung carcinoma cell lines. Oncogene 16: 153–165.
Freije JM, Fraile JM, Lopez-Otin C . (2011). Protease addiction and synthetic lethality in cancer. Front Oncol 1 (doi:10.3389/fonc.2011.00025).
Gao J, Sun L, Huo L, Liu M, Li D, Zhou J . (2010). CYLD regulates angiogenesis by mediating vascular endothelial cell migration. Blood 115: 4130–4137.
Gilbert RE, Huang Q, Thai K, Advani SL, Lee K, Yuen DA et al. (2011). Histone deacetylase inhibition attenuates diabetes-associated kidney growth: potential role for epigenetic modification of the epidermal growth factor receptor. Kidney Int 79: 1312–1321.
Glinsky GV . (2005). Death-from-cancer signatures and stem cell contribution to metastatic cancer. Cell Cycle 4: 1171–1175.
Gonzalez-Navajas JM, Law J, Nguyen KP, Bhargava M, Corr MP, Varki N et al. (2010). Interleukin 1 receptor signaling regulates DUBA expression and facilitates Toll-like receptor 9-driven antiinflammatory cytokine production. J Exp Med 207: 2799–2807.
Graner E, Tang D, Rossi S, Baron A, Migita T, Weinstein LJ et al. (2004). The isopeptidase USP2a regulates the stability of fatty acid synthase in prostate cancer. Cancer Cell 5: 253–261.
Gray DA, Inazawa J, Gupta K, Wong A, Ueda R, Takahashi T . (1995). Elevated expression of Unph, a proto-oncogene at 3p21.3, in human lung tumors. Oncogene 10: 2179–2183.
Guedat P, Colland F . (2007). Patented small molecule inhibitors in the ubiquitin proteasome system. BMC Biochem 8 (Suppl 1): S14.
Guervilly JH, Renaud E, Takata M, Rosselli F . (2011). USP1 deubiquitinase maintains phosphorylated CHK1 by limiting its DDB1-dependent degradation. Hum Mol Genet 20: 2171–2181.
Gupta K, Chevrette M, Gray DA . (1994). The Unp proto-oncogene encodes a nuclear protein. Oncogene 9: 1729–1731.
Guterman A, Glickman MH . (2004a). Complementary roles for Rpn11 and Ubp6 in deubiquitination and proteolysis by the proteasome. J Biol Chem 279: 1729–1738.
Guterman A, Glickman MH . (2004b). Deubiquitinating enzymes are IN/(trinsic to proteasome function). Curr Protein Pept Sci 5: 201–211.
Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA et al. (2010). Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 330: 1410–1413.
Harhaj EW, Dixit VM . (2011). Deubiquitinases in the regulation of NF-kappaB signaling. Cell Res 21: 22–39.
Harris SL, Levine AJ . (2005). The p53 pathway: positive and negative feedback loops. Oncogene 24: 2899–2908.
Hartwell LH, Szankasi P, Roberts CJ, Murray AW, Friend SH . (1997). Integrating genetic approaches into the discovery of anticancer drugs. Science 278: 1064–1068.
Hellerbrand C, Bumes E, Bataille F, Dietmaier W, Massoumi R, Bosserhoff AK . (2007). Reduced expression of CYLD in human colon and hepatocellular carcinomas. Carcinogenesis 28: 21–27.
Hershko A, Heller H, Elias S, Ciechanover A . (1983). Components of ubiquitin-protein ligase system. Resolution, affinity purification, and role in protein breakdown. J Biol Chem 258: 8206–8214.
Hirayama K, Aoki S, Nishikawa K, Matsumoto T, Wada K . (2007). Identification of novel chemical inhibitors for ubiquitin C-terminal hydrolase-L3 by virtual screening. Bioorg Med Chem 15: 6810–6818.
Honma K, Tsuzuki S, Nakagawa M, Tagawa H, Nakamura S, Morishima Y et al. (2009). TNFAIP3/A20 functions as a novel tumor suppressor gene in several subtypes of non-Hodgkin lymphomas. Blood 114: 2467–2475.
Hu M, Li P, Li M, Li W, Yao T, Wu JW et al. (2002). Crystal structure of a UBP-family deubiquitinating enzyme in isolation and in complex with ubiquitin aldehyde. Cell 111: 1041–1054.
Hu M, Li P, Song L, Jeffrey PD, Chenova TA, Wilkinson KD et al. (2005). Structure and mechanisms of the proteasome-associated deubiquitinating enzyme USP14. EMBO J 24: 3747–3756.
Huang TT, Nijman SM, Mirchandani KD, Galardy PJ, Cohn MA, Haas W et al. (2006). Regulation of monoubiquitinated PCNA by DUB autocleavage. Nat Cell Biol 8: 339–347.
Huang X, Langelotz C, Hetfeld-Pechoc BK, Schwenk W, Dubiel W . (2009). The COP9 signalosome mediates beta-catenin degradation by deneddylation and blocks adenomatous polyposis coli destruction via USP15. J Mol Biol 391: 691–702.
Huang X, Summers MK, Pham V, Lill JR, Liu J, Lee G et al. (2011). Deubiquitinase USP37 is activated by CDK2 to antagonize APC(CDH1) and promote S phase entry. Mol Cell 42: 511–523.
Hussain S, Foreman O, Perkins SL, Witzig TE, Miles RR, van Deursen J et al. (2010). The de-ubiquitinase UCH-L1 is an oncogene that drives the development of lymphoma in vivo by deregulating PHLPP1 and Akt signaling. Leukemia 24: 1641–1655.
Hussain S, Zhang Y, Galardy PJ . (2009). DUBs and cancer: the role of deubiquitinating enzymes as oncogenes, non-oncogenes and tumor suppressors. Cell Cycle 8: 1688–1697.
Hymowitz SG, Wertz IE . (2010). A20: from ubiquitin editing to tumour suppression. Nat Rev Cancer 10: 332–341.
Ibarrola N, Kratchmarova I, Nakajima D, Schiemann WP, Moustakas A, Pandey A et al. (2004). Cloning of a novel signaling molecule, AMSH-2, that potentiates transforming growth factor beta signaling. BMC Cell Biol 5: 2.
Ikeda F, Dikic I . (2008). Atypical ubiquitin chains: new molecular signals. ‘Protein Modifications: Beyond the Usual Suspects’ review series. EMBO Rep 9: 536–542.
Jang MJ, Baek SH, Kim JH . (2011). UCH-L1 promotes cancer metastasis in prostate cancer cells through EMT induction. Cancer Lett 302: 128–135.
Jaster R, Baek KH, D'Andrea AD . (1999). Analysis of cis-acting sequences and trans-acting factors regulating the interleukin-3 response element of the DUB-1 gene. Biochim Biophys Acta 1446: 308–316.
Jenner MW, Leone PE, Walker BA, Ross FM, Johnson DC, Gonzalez D et al. (2007). Gene mapping and expression analysis of 16q loss of heterozygosity identifies WWOX and CYLD as being important in determining clinical outcome in multiple myeloma. Blood 110: 3291–3300.
Jensen DE, Proctor M, Marquis ST, Gardner HP, Ha SI, Chodosh LA et al. (1998). BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene 16: 1097–1112.
Joo HY, Zhai L, Yang C, Nie S, Erdjument-Bromage H, Tempst P et al. (2007). Regulation of cell cycle progression and gene expression by H2A deubiquitination. Nature 449: 1068–1072.
Kaelin Jr WG . (2005). The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer 5: 689–698.
Kapuria V, Peterson LF, Fang D, Bornmann WG, Talpaz M, Donato NJ . (2010). Deubiquitinase inhibition by small-molecule WP1130 triggers aggresome formation and tumor cell apoptosis. Cancer Res 70: 9265–9276.
Kessler BM, Edelmann MJ . (2011). PTMs in conversation: activity and function of deubiquitinating enzymes regulated via post-translational modifications. Cell Biochem Biophys 60: 21–38.
Kim MS, Kim YK, Kim YS, Seong M, Choi JK, Baek KH . (2005). Deubiquitinating enzyme USP36 contains the PEST motif and is polyubiquitinated. Biochem Biophys Res Commun 330: 797–804.
Klaus A, Birchmeier W . (2008). Wnt signalling and its impact on development and cancer. Nat Rev Cancer 8: 387–398.
Komander D, Clague MJ, Urbe S . (2009a). Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol 10: 550–563.
Komander D, Lord CJ, Scheel H, Swift S, Hofmann K, Ashworth A et al. (2008). The structure of the CYLD USP domain explains its specificity for Lys63-linked polyubiquitin and reveals a B box module. Mol Cell 29: 451–464.
Komander D, Reyes-Turcu F, Licchesi JD, Odenwaelder P, Wilkinson KD, Barford D . (2009b). Molecular discrimination of structurally equivalent Lys 63-linked and linear polyubiquitin chains. EMBO Rep 10: 466–473.
Kon N, Kobayashi Y, Li M, Brooks CL, Ludwig T, Gu W . (2010). Inactivation of HAUSP in vivo modulates p53 function. Oncogene 29: 1270–1279.
Koulich E, Li X, DeMartino GN . (2008). Relative structural and functional roles of multiple deubiquitylating proteins associated with mammalian 26S proteasome. Mol Biol Cell 19: 1072–1082.
Kuhlbrodt K, Janiesch PC, Kevei E, Segref A, Barikbin R, Hoppe T . (2011). The Machado-Joseph disease deubiquitylase ATX-3 couples longevity and proteostasis. Nat Cell Biol 13: 273–281.
Kuphal S, Shaw-Hallgren G, Eberl M, Karrer S, Aberger F, Bosserhoff AK et al. (2011). GLI1-dependent transcriptional repression of CYLD in basal cell carcinoma. Oncogene 30: 4523–4530.
Lee BH, Lee MJ, Park S, Oh DC, Elsasser S, Chen PC et al. (2010). Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature 467: 179–184.
Lee HJ, Kim MS, Kim YK, Oh YK, Baek KH . (2005). HAUSP, a deubiquitinating enzyme for p53, is polyubiquitinated, polyneddylated, and dimerized. FEBS Lett 579: 4867–4872.
Li L, Soetandyo N, Wang Q, Ye Y . (2009). The zinc finger protein A20 targets TRAF2 to the lysosomes for degradation. Biochim Biophys Acta 1793: 346–353.
Li Z, Na X, Wang D, Schoen SR, Messing EM, Wu G . (2002a). Ubiquitination of a novel deubiquitinating enzyme requires direct binding to von Hippel-Lindau tumor suppressor protein. J Biol Chem 277: 4656–4662.
Li Z, Wang D, Messing EM, Wu G . (2005). VHL protein-interacting deubiquitinating enzyme 2 deubiquitinates and stabilizes HIF-1alpha. EMBO Rep 6: 373–378.
Li Z, Wang D, Na X, Schoen SR, Messing EM, Wu G . (2002b). Identification of a deubiquitinating enzyme subfamily as substrates of the von Hippel-Lindau tumor suppressor. Biochem Biophys Res Commun 294: 700–709.
Liang J, Saad Y, Lei T, Wang J, Qi D, Yang Q et al. (2010). MCP-induced protein 1 deubiquitinates TRAF proteins and negatively regulates JNK and NF-kappaB signaling. J Exp Med 207: 2959–2973.
Liu H, Buus R, Clague MJ, Urbe S . (2009). Regulation of ErbB2 receptor status by the proteasomal DUB POH1. PLoS One 4: e5544.
Liu J, Chung HJ, Vogt M, Jin Y, Malide D, He L et al. (2011). JTV1 co-activates FBP to induce USP29 transcription and stabilize p53 in response to oxidative stress. EMBO J 30: 846–858.
Liu Y, Lashuel HA, Choi S, Xing X, Case A, Ni J et al. (2003). Discovery of inhibitors that elucidate the role of UCH-L1 activity in the H1299 lung cancer cell line. Chem Biol 10: 837–846.
Lopez-Otin C, Bond JS . (2008). Proteases: multifunctional enzymes in life and disease. J Biol Chem 283: 30433–30437.
Lopez-Otin C, Hunter T . (2010). The regulatory crosstalk between kinases and proteases in cancer. Nat Rev Cancer 10: 278–292.
Lopez-Otin C, Overall CM . (2002). Protease degradomics: a new challenge for proteomics. Nat Rev Mol Cell Biol 3: 509–519.
Love KR, Catic A, Schlieker C, Ploegh HL . (2007). Mechanisms, biology and inhibitors of deubiquitinating enzymes. Nat Chem Biol 3: 697–705.
Lu Y, Adegoke OA, Nepveu A, Nakayama KI, Bedard N, Cheng D et al. (2009). USP19 deubiquitinating enzyme supports cell proliferation by stabilizing KPC1, a ubiquitin ligase for p27Kip1. Mol Cell Biol 29: 547–558.
Lu Y, Bedard N, Chevalier S, Wing SS . (2011). Identification of distinctive patterns of USP19-mediated growth regulation in normal and malignant cells. PLoS One 6: e15936.
Lui TT, Lacroix C, Ahmed SM, Goldenberg SJ, Leach CA, Daulat AM et al. (2011). The ubiquitin-specific protease USP34 regulates axin stability and Wnt/{beta}-catenin signaling. Mol Cell Biol 31: 2053–2065.
Luise C, Capra M, Donzelli M, Mazzarol G, Jodice MG, Nuciforo P et al. (2011). An atlas of altered expression of deubiquitinating enzymes in human cancer. PLoS One 6: e15891.
Luna-Vargas MP, Faesen AC, van Dijk WJ, Rape M, Fish A, Sixma TK . (2011). Ubiquitin-specific protease 4 is inhibited by its ubiquitin-like domain. EMBO Rep 12: 365–372.
Maertens GN, El Messaoudi-Aubert S, Elderkin S, Hiom K, Peters G . (2010). Ubiquitin-specific proteases 7 and 11 modulate Polycomb regulation of the INK4a tumour suppressor. EMBO J 29: 2553–2565.
Makarova KS, Aravind L, Koonin EV . (2000). A novel superfamily of predicted cysteine proteases from eukaryotes, viruses and Chlamydia pneumoniae. Trends Biochem Sci 25: 50–52.
Mao Y, Senic-Matuglia F, Di Fiore PP, Polo S, Hodsdon ME, De Camilli P . (2005). Deubiquitinating function of ataxin-3: insights from the solution structure of the Josephin domain. Proc Natl Acad Sci USA 102: 12700–12705.
Mason SD, Joyce JA . (2011). Proteolytic networks in cancer. Trends Cell Biol 21: 228–237.
Massoumi R, Chmielarska K, Hennecke K, Pfeifer A, Fassler R . (2006). Cyld inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-kappaB signaling. Cell 125: 665–677.
Massoumi R, Kuphal S, Hellerbrand C, Haas B, Wild P, Spruss T et al. (2009). Down-regulation of CYLD expression by Snail promotes tumor progression in malignant melanoma. J Exp Med 206: 221–232.
Mauri PL, Riva M, Ambu D, De Palma A, Secundo F, Benazzi L et al. (2006). Ataxin-3 is subject to autolytic cleavage. FEBS J 273: 4277–4286.
McCullough J, Clague MJ, Urbe S . (2004). AMSH is an endosome-associated ubiquitin isopeptidase. J Cell Biol 166: 487–492.
McFarlane C, Kelvin AA, de la Vega M, Govender U, Scott CJ, Burrows JF et al. (2010). The deubiquitinating enzyme USP17 is highly expressed in tumor biopsies, is cell cycle regulated, and is required for G1-S progression. Cancer Res 70: 3329–3339.
McNally RS, Davis BK, Clements CM, Accavitti-Loper MA, Mak TW, Ting JP . (2011). DJ-1 enhances cell survival through the binding of Cezanne, a negative regulator of NF-kappaB. J Biol Chem 286: 4098–4106.
Meray RK, Lansbury Jr PT . (2007). Reversible monoubiquitination regulates the Parkinson disease-associated ubiquitin hydrolase UCH-L1. J Biol Chem 282: 10567–10575.
Metzig M, Nickles D, Falschlehner C, Lehmann-Koch J, Straub BK, Roth W et al. (2011). An RNAi screen identifies USP2 as a factor required for TNF-alpha-induced NF-kappaB signaling. Int J Cancer 129: 607–618.
Meulmeester E, Kunze M, Hsiao HH, Urlaub H, Melchior F . (2008). Mechanism and consequences for paralog-specific sumoylation of ubiquitin-specific protease 25. Mol Cell 30: 610–619.
Mosesson Y, Mills GB, Yarden Y . (2008). Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer 8: 835–850.
Mullally JE, Fitzpatrick FA . (2002). Pharmacophore model for novel inhibitors of ubiquitin isopeptidases that induce p53-independent cell death. Mol Pharmacol 62: 351–358.
Murai J, Yang K, Dejsuphong D, Hirota K, Takeda S, D'Andrea AD . (2011). The USP1/UAF1 complex promotes double-strand break repair through homologous recombination. Mol Cell Biol 31: 2462–2469.
Nagai H, Noguchi T, Homma K, Katagiri K, Takeda K, Matsuzawa A et al. (2009). Ubiquitin-like sequence in ASK1 plays critical roles in the recognition and stabilization by USP9X and oxidative stress-induced cell death. Mol Cell 36: 805–818.
Nakamura N, Hirose S . (2008). Regulation of mitochondrial morphology by USP30, a deubiquitinating enzyme present in the mitochondrial outer membrane. Mol Biol Cell 19: 1903–1911.
Navon A, Ciechanover A . (2009). The 26 S proteasome: from basic mechanisms to drug targeting. J Biol Chem 284: 33713–33718.
Nicastro G, Menon RP, Masino L, Knowles PP, McDonald NQ, Pastore A . (2005). The solution structure of the Josephin domain of ataxin-3: structural determinants for molecular recognition. Proc Natl Acad Sci USA 102: 10493–10498.
Niendorf S, Oksche A, Kisser A, Lohler J, Prinz M, Schorle H et al. (2007). Essential role of ubiquitin-specific protease 8 for receptor tyrosine kinase stability and endocytic trafficking in vivo. Mol Cell Biol 27: 5029–5039.
Nijman SM, Huang TT, Dirac AM, Brummelkamp TR, Kerkhoven RM, D'Andrea AD et al. (2005). The deubiquitinating enzyme USP1 regulates the Fanconi anemia pathway. Mol Cell 17: 331–339.
Novak U, Rinaldi A, Kwee I, Nandula SV, Rancoita PM, Compagno M et al. (2009). The NF-{kappa}B negative regulator TNFAIP3 (A20) is inactivated by somatic mutations and genomic deletions in marginal zone lymphomas. Blood 113: 4918–4921.
Oh KH, Yang SW, Park JM, Seol JH, Iemura S, Natsume T et al. (2011). Control of AIF-mediated cell death by antagonistic functions of CHIP ubiquitin E3 ligase and USP2 deubiquitinating enzyme. Cell Death Differ 18: 1326–1336.
Oliveira AM, Hsi BL, Weremowicz S, Rosenberg AE, Dal Cin P, Joseph N et al. (2004). USP6 (Tre2) fusion oncogenes in aneurysmal bone cyst. Cancer Res 64: 1920–1923.
Pardali K, Moustakas A . (2007). Actions of TGF-beta as tumor suppressor and pro-metastatic factor in human cancer. Biochim Biophys Acta 1775: 21–62.
Parsons JL, Dianova II, Khoronenkova SV, Edelmann MJ, Kessler BM, Dianov GL . (2011). USP47 is a deubiquitylating enzyme that regulates base excision repair by controlling steady-state levels of DNA polymerase beta. Mol Cell 41: 609–615.
Pereg Y, Liu BY, O'Rourke KM, Sagolla M, Dey A, Komuves L et al. (2010). Ubiquitin hydrolase Dub3 promotes oncogenic transformation by stabilizing Cdc25A. Nat Cell Biol 12: 400–406.
Pickart CM, Rose IA . (1985). Ubiquitin carboxyl-terminal hydrolase acts on ubiquitin carboxyl-terminal amides. J Biol Chem 260: 7903–7910.
Popov N, Wanzel M, Madiredjo M, Zhang D, Beijersbergen R, Bernards R et al. (2007). The ubiquitin-specific protease USP28 is required for MYC stability. Nat Cell Biol 9: 765–774.
Prasad S, Ravindran J, Aggarwal BB . (2010). NF-kappaB and cancer: how intimate is this relationship. Mol Cell Biochem 336: 25–37.
Priolo C, Tang D, Brahamandan M, Benassi B, Sicinska E, Ogino S et al. (2006). The isopeptidase USP2a protects human prostate cancer from apoptosis. Cancer Res 66: 8625–8632.
Puente XS, Lopez-Otin C . (2004). A genomic analysis of rat proteases and protease inhibitors. Genome Res 14: 609–622.
Puente XS, Sanchez LM, Overall CM, Lopez-Otin C . (2003). Human and mouse proteases: a comparative genomic approach. Nat Rev Genet 4: 544–558.
Quesada V, Diaz-Perales A, Gutierrez-Fernandez A, Garabaya C, Cal S, Lopez-Otin C . (2004). Cloning and enzymatic analysis of 22 novel human ubiquitin-specific proteases. Biochem Biophys Res Commun 314: 54–62.
Quesada V, Ordonez GR, Sanchez LM, Puente XS, Lopez-Otin C . (2009). The Degradome database: mammalian proteases and diseases of proteolysis. Nucleic Acids Res 37: D239–D243.
Rajan N, Elliott R, Clewes O, Mackay A, Reis-Filho JS, Burn J et al. (2011). Dysregulated TRK signalling is a therapeutic target in CYLD defective tumours. Oncogene 30: 4243–4260.
Ramakrishna S, Suresh B, Baek KH . (2011a). The role of deubiquitinating enzymes in apoptosis. Cell Mol Life Sci 68: 15–26.
Ramakrishna S, Suresh B, Lee EJ, Lee HJ, Ahn WS, Baek KH . (2011b). Lys-63-specific deubiquitination of SDS3 by USP17 regulates HDAC activity. J Biol Chem 286: 10505–10514.
Rehman FL, Lord CJ, Ashworth A . (2010). Synthetic lethal approaches to breast cancer therapy. Nat Rev Clin Oncol 7: 718–724.
Reiley W, Zhang M, Sun SC . (2004). Negative regulation of JNK signaling by the tumor suppressor CYLD. J Biol Chem 279: 55161–55167.
Reiley WW, Jin W, Lee AJ, Wright A, Wu X, Tewalt EF et al. (2007). Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses. J Exp Med 204: 1475–1485.
Reyes-Turcu FE, Ventii KH, Wilkinson KD . (2009). Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem 78: 363–397.
Saggar S, Chernoff KA, Lodha S, Horev L, Kohl S, Honjo RS et al. (2008). CYLD mutations in familial skin appendage tumours. J Med Genet 45: 298–302.
Sato Y, Yoshikawa A, Yamagata A, Mimura H, Yamashita M, Ookata K et al. (2008). Structural basis for specific cleavage of Lys 63-linked polyubiquitin chains. Nature 455: 358–362.
Schoenfeld AR, Apgar S, Dolios G, Wang R, Aaronson SA . (2004). BRCA2 is ubiquitinated in vivo and interacts with USP11, a deubiquitinating enzyme that exhibits prosurvival function in the cellular response to DNA damage. Mol Cell Biol 24: 7444–7455.
Schweitzer K, Bozko PM, Dubiel W, Naumann M . (2007). CSN controls NF-kappaB by deubiquitinylation of IkappaBalpha. EMBO J 26: 1532–1541.
Schwickart M, Huang X, Lill JR, Liu J, Ferrando R, French DM et al. (2010). Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature 463: 103–107.
Shah SP, Morin RD, Khattra J, Prentice L, Pugh T, Burleigh A et al. (2009). Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature 461: 809–813.
Shan J, Zhao W, Gu W . (2009). Suppression of cancer cell growth by promoting cyclin D1 degradation. Mol Cell 36: 469–476.
Solimini NL, Luo J, Elledge SJ . (2007). Non-oncogene addiction and the stress phenotype of cancer cells. Cell 130: 986–988.
Song MS, Salmena L, Carracedo A, Egia A, Lo-Coco F, Teruya-Feldstein J et al. (2008). The deubiquitinylation and localization of PTEN are regulated by a HAUSP-PML network. Nature 455: 813–817.
Sowa ME, Bennett EJ, Gygi SP, Harper JW . (2009). Defining the human deubiquitinating enzyme interaction landscape. Cell 138: 389–403.
Staal J, Driege Y, Bekaert T, Demeyer A, Muyllaert D, Van Damme P et al. (2011). T-cell receptor-induced JNK activation requires proteolytic inactivation of CYLD by MALT1. EMBO J 30: 1742–1752.
Stegmeier F, Rape M, Draviam VM, Nalepa G, Sowa ME, Ang XL et al. (2007a). Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature 446: 876–881.
Stegmeier F, Sowa ME, Nalepa G, Gygi SP, Harper JW, Elledge SJ . (2007b). The tumor suppressor CYLD regulates entry into mitosis. Proc Natl Acad Sci USA 104: 8869–8874.
Stevenson LF, Sparks A, Allende-Vega N, Xirodimas DP, Lane DP, Saville MK . (2007). The deubiquitinating enzyme USP2a regulates the p53 pathway by targeting Mdm2. EMBO J 26: 976–986.
Sun H, Kapuria V, Peterson LF, Fang D, Bornmann WG, Bartholomeusz G et al. (2011). Bcr-Abl ubiquitination and Usp9x inhibition block kinase signaling and promote CML cell apoptosis. Blood 117: 3151–3162.
Tauriello DV, Haegebarth A, Kuper I, Edelmann MJ, Henraat M, Canninga-van Dijk MR et al. (2010). Loss of the tumor suppressor CYLD enhances Wnt/beta-catenin signaling through K63-linked ubiquitination of Dvl. Mol Cell 37: 607–619.
Tian X, Isamiddinova NS, Peroutka RJ, Goldenberg SJ, Mattern MR, Nicholson B et al. (2011). Characterization of selective ubiquitin and ubiquitin-like protease inhibitors using a fluorescence-based multiplex assay format. Assay Drug Dev Technol 9: 165–173.
Todi SV, Winborn BJ, Scaglione KM, Blount JR, Travis SM, Paulson HL . (2009). Ubiquitination directly enhances activity of the deubiquitinating enzyme ataxin-3. EMBO J 28: 372–382.
Tran H, Hamada F, Schwarz-Romond T, Bienz M . (2008). Trabid, a new positive regulator of Wnt-induced transcription with preference for binding and cleaving K63-linked ubiquitin chains. Genes Dev 22: 528–542.
Turk B . (2006). Targeting proteases: successes, failures and future prospects. Nat Rev Drug Discov 5: 785–799.
Urbanik T, Kohler BC, Boger RJ, Worns MA, Heeger S, Otto G et al. (2011). Down-regulation of CYLD as a trigger for NF-kappaB activation and a mechanism of apoptotic resistance in hepatocellular carcinoma cells. Int J Oncol 38: 121–131.
van der Horst A, de Vries-Smits AM, Brenkman AB, van Triest MH, van den Broek N, Colland F et al. (2006). FOXO4 transcriptional activity is regulated by monoubiquitination and USP7/HAUSP. Nat Cell Biol 8: 1064–1073.
van Leuken RJ, Luna-Vargas MP, Sixma TK, Wolthuis RM, Medema RH . (2008). Usp39 is essential for mitotic spindle checkpoint integrity and controls mRNA-levels of aurora B. Cell Cycle 7: 2710–2719.
Vendrell JA, Ghayad S, Ben-Larbi S, Dumontet C, Mechti N, Cohen PA . (2007). A20/TNFAIP3, a new estrogen-regulated gene that confers tamoxifen resistance in breast cancer cells. Oncogene 26: 4656–4667.
Ventii KH, Wilkinson KD . (2008). Protein partners of deubiquitinating enzymes. Biochem J 414: 161–175.
Verstrepen L, Carpentier I, Verhelst K, Beyaert R . (2009). ABINs: A20 binding inhibitors of NF-kappa B and apoptosis signaling. Biochem Pharmacol 78: 105–114.
Virdee S, Ye Y, Nguyen DP, Komander D, Chin JW . (2010). Engineered diubiquitin synthesis reveals Lys29-isopeptide specificity of an OTU deubiquitinase. Nat Chem Biol 6: 750–757.
Vogelstein B, Kinzler KW . (2004). Cancer genes and the pathways they control. Nat Med 10: 789–799.
Vucic D, Dixit VM, Wertz IE . (2011). Ubiquitylation in apoptosis: a post-translational modification at the edge of life and death. Nat Rev Mol Cell Biol 12: 439–452.
Wertz IE, O'Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S et al. (2004). De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature 430: 694–699.
Wicks SJ, Haros K, Maillard M, Song L, Cohen RE, Dijke PT et al. (2005). The deubiquitinating enzyme UCH37 interacts with Smads and regulates TGF-beta signalling. Oncogene 24: 8080–8084.
Wilkinson KD . (1997). Regulation of ubiquitin-dependent processes by deubiquitinating enzymes. FASEB J 11: 1245–1256.
Winborn BJ, Travis SM, Todi SV, Scaglione KM, Xu P, Williams AJ et al. (2008). The deubiquitinating enzyme ataxin-3, a polyglutamine disease protein, edits Lys63 linkages in mixed linkage ubiquitin chains. J Biol Chem 283: 26436–26443.
Xu G, Tan X, Wang H, Sun W, Shi Y, Burlingame S et al. (2010). Ubiquitin-specific peptidase 21 inhibits tumor necrosis factor alpha-induced nuclear factor kappaB activation via binding to and deubiquitinating receptor-interacting protein 1. J Biol Chem 285: 969–978.
Xu M, Takanashi M, Oikawa K, Tanaka M, Nishi H, Isaka K et al. (2009). USP15 plays an essential role for caspase-3 activation during Paclitaxel-induced apoptosis. Biochem Biophys Res Commun 388: 366–371.
Yamaguchi T, Kimura J, Miki Y, Yoshida K . (2007). The deubiquitinating enzyme USP11 controls an IkappaB kinase alpha (IKKalpha)-p53 signaling pathway in response to tumor necrosis factor alpha (TNFalpha). J Biol Chem 282: 33943–33948.
Yang Y, Hou JQ, Qu LY, Wang GQ, Ju HW, Zhao ZW et al. (2007). [Differential expression of USP2, USP14 and UBE4A between ovarian serous cystadenocarcinoma and adjacent normal tissues]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 23: 504–506.
Yao T, Song L, Jin J, Cai Y, Takahashi H, Swanson SK et al. (2008). Distinct modes of regulation of the Uch37 deubiquitinating enzyme in the proteasome and in the Ino80 chromatin-remodeling complex. Mol Cell 31: 909–917.
Yoshida A, Yoneda-Kato N, Panattoni M, Pardi R, Kato JY . (2010). CSN5/Jab1 controls multiple events in the mammalian cell cycle. FEBS Lett 584: 4545–4552.
Yoshida H, Jono H, Kai H, Li JD . (2005). The tumor suppressor cylindromatosis (CYLD) acts as a negative regulator for toll-like receptor 2 signaling via negative cross-talk with TRAF6 AND TRAF7. J Biol Chem 280: 41111–41121.
Yu H, Mashtalir N, Daou S, Hammond-Martel I, Ross J, Sui G et al. (2010). The ubiquitin carboxyl hydrolase BAP1 forms a ternary complex with YY1 and HCF-1 and is a critical regulator of gene expression. Mol Cell Biol 30: 5071–5085.
Yu J, Tao Q, Cheung KF, Jin H, Poon FF, Wang X et al. (2008). Epigenetic identification of ubiquitin carboxyl-terminal hydrolase L1 as a functional tumor suppressor and biomarker for hepatocellular carcinoma and other digestive tumors. Hepatology 48: 508–518.
Yuan J, Luo K, Zhang L, Cheville JC, Lou Z . (2010). USP10 regulates p53 localization and stability by deubiquitinating p53. Cell 140: 384–396.
Zhang C, Cai TY, Zhu H, Yang LQ, Jiang H, Dong XW et al. (2011a). Synergistic antitumor activity of gemcitabine and ABT-737 in vitro and in vivo through disrupting the interaction of USP9X and Mcl-1. Mol Cancer Ther 10: 1264–1275.
Zhang D, Zaugg K, Mak TW, Elledge SJ . (2006a). A role for the deubiquitinating enzyme USP28 in control of the DNA-damage response. Cell 126: 529–542.
Zhang J, Stirling B, Temmerman ST, Ma CA, Fuss IJ, Derry JM et al. (2006b). Impaired regulation of NF-kappaB and increased susceptibility to colitis-associated tumorigenesis in CYLD-deficient mice. J Clin Invest 116: 3042–3049.
Zhang X, Berger FG, Yang J, Lu X . (2011b). USP4 inhibits p53 through deubiquitinating and stabilizing ARF-BP1. EMBO J 30: 2177–2189.
Zhang XY, Varthi M, Sykes SM, Phillips C, Warzecha C, Zhu W et al. (2008). The putative cancer stem cell marker USP22 is a subunit of the human SAGA complex required for activated transcription and cell-cycle progression. Mol Cell 29: 102–111.
Zhang Y . (2003). Transcriptional regulation by histone ubiquitination and deubiquitination. Genes Dev 17: 2733–2740.
Zhao B, Schlesiger C, Masucci MG, Lindsten K . (2009). The ubiquitin specific protease 4 (USP4) is a new player in the Wnt signalling pathway. J Cell Mol Med 13: 1886–1895.
Zhu P, Zhou W, Wang J, Puc J, Ohgi KA, Erdjument-Bromage H et al. (2007a). A histone H2A deubiquitinase complex coordinating histone acetylation and H1 dissociation in transcriptional regulation. Mol Cell 27: 609–621.
Zhu X, Menard R, Sulea T . (2007b). High incidence of ubiquitin-like domains in human ubiquitin-specific proteases. Proteins 69: 1–7.
Acknowledgements
Our study is supported by grants from Ministerio de Ciencia e Innovación-Spain, Fundación ‘M Botín’, PCTI-FICYT Asturias and European Union (FP7 MicroEnviMet). The Instituto Universitario de Oncología is supported by Obra Social Cajastur and Acción Transversal del Cáncer-RTICC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Fraile, J., Quesada, V., Rodríguez, D. et al. Deubiquitinases in cancer: new functions and therapeutic options. Oncogene 31, 2373–2388 (2012). https://doi.org/10.1038/onc.2011.443
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.443
Keywords
This article is cited by
-
Josephin domain containing 2 (JOSD2) promotes lung cancer by inhibiting LKB1 (Liver kinase B1) activity
Signal Transduction and Targeted Therapy (2024)
-
Deubiquitinase YOD1 suppresses tumor progression by stabilizing E3 ligase TRIM33 in head and neck squamous cell carcinoma
Cell Death & Disease (2023)
-
Targeting USP10 induces degradation of oncogenic ANLN in esophageal squamous cell carcinoma
Cell Death & Differentiation (2023)
-
miR-221/222 induce instability of p53 By downregulating deubiquitinase YOD1 in acute myeloid leukemia
Cell Death Discovery (2023)
-
MFG-E8 stabilized by deubiquitinase USP14 suppresses cigarette smoke-induced ferroptosis in bronchial epithelial cells
Cell Death & Disease (2023)