Abstract
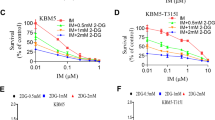
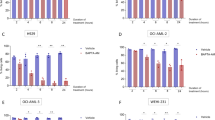
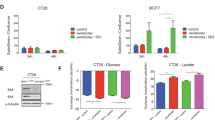
Targeting altered cancer cell metabolism with the glycolysis inhibitor, 2-deoxyglucose (2DG), is a viable therapeutic strategy, but the effects of 2DG on lymphoma cells and the mechanism of action are unknown. Five T-cell lymphoma lines and two B-cell lymphoma lines were shown to be highly sensitive to 2DG. Examination of the cell death pathway demonstrated pro-apoptotic protein Bax ‘activation’ and caspase cleavage in 2DG-treated cells. However, Q-VD-OPh, a potent inhibitor of caspase activity provided minimal protection from death. In contrast, overexpressing the anti-apoptotic protein Bcl-2 dramatically enhanced the survival of 2DG-treated cells that was negated by a Bcl-2 antagonist. BH3-only members, Bim and Bmf, were upregulated by 2DG, and shRNAs targeting Bim protected from 2DG toxicity demonstrating that Bim is a critical mediator of 2DG toxicity. 2DG also induced GADD153/CHOP expression, a marker of endoplasmic reticulum (ER) stress and a known activator of Bim. Mannose, a reagent known to alleviate ER stress, transiently protected from 2DG-induced cell death. Examination of the effects of 2DG on energy metabolism showed a drop in ATP levels by 30 min that was not affected by either Bcl-2 or mannose. These results demonstrate that ER stress appears to be rate limiting in 2DG-induced cell death in lymphoma cells, and this cell killing is regulated by the Bcl-2 family of proteins. Bcl-2 inhibition combined with 2DG may be an effective therapeutic strategy for lymphoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Adams JM, Cory S . (1998). The bcl-2 protein family: arbiters of cell survival. Science 281: 1322–1326.
Aykin-Burns N, Ahmad IM, Zhu Y, Oberley LW, Spitz DR . (2009). Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem J 418: 29–37.
Ben Sahra I, Laurent K, Giuliano S, Larbret F, Ponzio G, Gounon P et al. (2010). Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res 70: 2465–2475.
Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM . (2004). Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 304: 596–600.
Chen C, Okayama H . (1987). High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol 7: 2745–2752.
Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG et al. (2005). Differential targeting of prosurvival bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 17: 393–403.
Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T et al. (2001). BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell 8: 705–711.
Chipuk JE, Green DR . (2005). Do inducers of apoptosis trigger caspase-independent cell death? Nat Rev Mol Cell Biol 6: 268–275.
Crane RK, Sols A . (1954). The non-competitive inhibition of brain hexokinase by glucose-6-phosphate and related compounds. J Biol Chem 210: 597–606.
Dasmahapatra G, Lembersky D, Rahmani M, Kramer L, Friedberg J, Fisher RI et al. (2009). Bcl-2 antagonists interact synergistically with bortezomib in DLBCL cells in association with JNK activation and induction of ER stress. Cancer Biol Ther 8: 808–819.
Egle A, Harris AW, Bouillet P, Cory S . (2004). Bim is a suppressor of myc-induced mouse B cell leukemia. Proc Natl Acad Sci USA 101: 6164–6169.
El Mjiyad N, Caro-Maldonado A, Ramirez-Peinado S, Munoz-Pinedo C . (2011). Sugar-free approaches to cancer cell killing. Oncogene 30: 253–264.
Gross A, McDonnell JM, Korsmeyer SJ . (1999). BCL-2 family members and the mitochondria in apoptosis. Genes Dev 13: 1899–1911.
Han SS, Peng L, Chung ST, DuBois W, Maeng SH, Shaffer AL et al. (2006). CDDO-imidazolide inhibits growth and survival of c-myc-induced mouse B cell and plasma cell neoplasms. Mol Cancer 5: 22.
Hsu YT, Youle RJ . (1998). Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem 273: 10777–10783.
Huang DC, Strasser A . (2000). BH3-only proteins-essential initiators of apoptotic cell death. Cell 103: 839–842.
Kane DJ, Ord T, Anton R, Bredesen DE . (1995). Expression of bcl-2 inhibits necrotic neural cell death. J Neurosci Res 40: 269–275.
Kang MH, Kang YH, Szymanska B, Wilczynska-Kalak U, Sheard MA, Harned TM et al. (2007). Activity of vincristine, L-ASP, and dexamethasone against acute lymphoblastic leukemia is enhanced by the BH3-mimetic ABT-737 in vitro and in vivo. Blood 110: 2057–2066.
Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N et al. (2002). Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol 22: 7405–7416.
Kurtoglu M, Gao N, Shang J, Maher JC, Lehrman MA, Wangpaichitr M et al. (2007). Under normoxia, 2-deoxy-D-glucose elicits cell death in select tumor types not by inhibition of glycolysis but by interfering with N-linked glycosylation. Mol Cancer Ther 6: 3049–3058.
Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR et al. (2005). BH3 domains of BH3-only proteins differentially regulate bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell 17: 525–535.
Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ . (2002). Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2: 183–192.
Letai A, Sorcinelli MD, Beard C, Korsmeyer SJ . (2004). Antiapoptotic BCL-2 is required for maintenance of a model leukemia. Cancer Cell 6: 241–249.
Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H et al. (1999). Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402: 672–676.
Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T et al. (2005). Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 120: 237–248.
Ma Y, Hendershot LM . (2004). ER chaperone functions during normal and stress conditions. J Chem Neuroanat 28: 51–65.
Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R et al. (2004). CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18: 3066–3077.
Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA et al. (2005). An inhibitor of bcl-2 family proteins induces regression of solid tumours. Nature 435: 677–681.
Oyadomari S, Mori M . (2004). Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11: 381–389.
Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND et al. (2007). ER stress triggers apoptosis by activating BH3-only protein bim. Cell 129: 1337–1349.
Raden D, Hildebrandt S, Xu P, Bell E, Doyle FJ, Robinson AS . (2005). Analysis of cellular response to protein overexpression. Syst Biol (Stevenage) 152: 285–289.
Rathmell JC, Fox CJ, Plas DR, Hammerman PS, Cinalli RM, Thompson CB . (2003). Akt-directed glucose metabolism can prevent bax conformation change and promote growth factor-independent survival. Mol Cell Biol 23: 7315–7328.
Reimertz C, Kogel D, Rami A, Chittenden T, Prehn JH . (2003). Gene expression during ER stress-induced apoptosis in neurons: induction of the BH3-only protein Bbc3/PUMA and activation of the mitochondrial apoptosis pathway. J Cell Biol 162: 587–597.
Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM et al. (2006). Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol 4: e374.
Saeki K, Yuo A, Okuma E, Yazaki Y, Susin SA, Kroemer G et al. (2000). Bcl-2 down-regulation causes autophagy in a caspase-independent manner in human leukemic HL60 cells. Cell Death Differ 7: 1263–1269.
Shoemaker AR, Oleksijew A, Bauch J, Belli BA, Borre T, Bruncko M et al. (2006). A small-molecule inhibitor of bcl-XL potentiates the activity of cytotoxic drugs in vitro and in vivo. Cancer Res 66: 8731–8739.
Simons AL, Ahmad IM, Mattson DM, Dornfeld KJ, Spitz DR . (2007). 2-deoxy-D-glucose combined with cisplatin enhances cytotoxicity via metabolic oxidative stress in human head and neck cancer cells. Cancer Res 67: 3364–3370.
Smaili SS, Hsu YT, Carvalho AC, Rosenstock TR, Sharpe JC, Youle RJ . (2003). Mitochondria, calcium and pro-apoptotic proteins as mediators in cell death signaling. Braz J Med Biol Res 36: 183–190.
Tower DB . (1958). The effects of 2-deoxy-D-glucose on metabolism of slices of cerebral cortex incubated in vitro. J Neurochem 3: 185–205.
van de Wetering CI, Horne MC, Knudson CM . (2007). Chromosomal instability and supernumerary centrosomes represent precursor defects in a mouse model of T-cell lymphoma. Cancer Res 67: 8081–8088.
van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE et al. (2006). The BH3 mimetic ABT-737 targets selective bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if mcl-1 is neutralized. Cancer Cell 10: 389–399.
Vander Heiden MG, Cantley LC, Thompson CB . (2009). Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324: 1029–1033.
Zhang XD, Deslandes E, Villedieu M, Poulain L, Duval M, Gauduchon P et al. (2006). Effect of 2-deoxy-D-glucose on various malignant cell lines in vitro. Anticancer Res 26: 3561–3566.
Acknowledgements
This work was supported by RO1 no. CA104695, RO1 no. CA133114, R01 no. DK084058, and R01 no. GM086389. We thank Dr Chris van de Wetering for assistance in generating lymphoma cell lines, Dr Siegfried Janz and Dr SS Han for B-cell neoplasms, Dr Agshin Taghiyev and Dr Van Tompkins for their advice and assistance with TCL transduction experiments, Dr Craig Kuder and Dr Ray Hohl for antibodies and assistance with ER-stress markers, Heather Tyra for assistance with RT–PCR, Han Du for advice with the Bcl-2 IP experiments, Rebecca Glover and Jacob Wolf for their technical advice on western blotting and Dr Craig Thompson (The Sloan-Kettering Cancer Center) for Bax/Bak double-knockout cells.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Zagorodna, O., Martin, S., Rutkowski, D. et al. 2-Deoxyglucose-induced toxicity is regulated by Bcl-2 family members and is enhanced by antagonizing Bcl-2 in lymphoma cell lines. Oncogene 31, 2738–2749 (2012). https://doi.org/10.1038/onc.2011.454
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.454