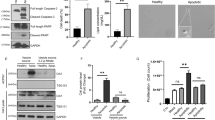
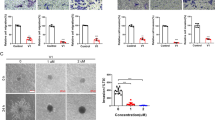
Abstract
Vesicular structures called microvesicles (MVs) that are shed from the surfaces of cancer cells are capable of transferring oncogenic cargo to recipient cancer cells, as well as to normal cells, sending mitogenic signals that greatly enhance tumor growth. Because MVs are stable in the circulation, they also may have a key role in secondary colonization and metastasis. Thus, understanding how MVs are generated could have important consequences for interfering with cancer progression. Here we report that the small GTPase RhoA triggers a specific signaling pathway essential for MV biogenesis in various human cancer cells. Inhibiting the activity of different proteins comprising this pathway blocks MV biogenesis in the donor cancer cells and prevents oncogenic transformation in cell culture as well as tumor growth in mice. Although RhoA has often been implicated in human cancer, these findings now highlight a previously unappreciated role for this GTPase in malignant transformation, and demonstrate that blocking MV biogenesis may offer novel approaches for interfering with malignant transformation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 2008; 10: 619–624.
Al-Nedawi K, Meehan B, Rak J . Microvesicles: messengers and mediators of tumor progression. Cell Cycle 2009; 8: 2014–2018.
Antonyak MA, Li B, Boroughs LK, Johnson JL, Druso JE, Bryant KL et al. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc Natl Acad Sci USA 2011; 108: 4852–4857.
Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 2008; 10: 1470–1476.
Lee TH, D'Asti E, Magnus N, Al-Nedawi K, Meehan B, Rak J . Microvesicles as mediators of intercellular communication in cancer-the emerging science of cellular ‘debris’. Semin Immunopathol 2011; 33: 455–467.
Rak J . Microparticles in cancer. Semin. Thromb Hemost 2010; 36: 888–906.
Keller S, Sanderson MP, Stoeck A, Altevogt P . Exosomes: from biogenesis and secretion to biological function. Immunol Lett 2006; 107: 102–108.
Cocucci E, Racchetti G, Meldolesi J . Shedding microvesicles: artefacts no more. Trends Cell Biol 2009; 19: 43–51.
Hao S, Ye Z, Li F, Meng Q, Qureshi M, Yang J et al. Epigenetic transfer of metastatic activity by uptake of highly metastatic B16 melanoma cell-released exosomes. Exp Oncol 2006; 28: 126–131.
Catellana D, Zobairi F, Martinez, MC, Panaro MA, Mitolo V, Freyssinet JM et al. Membrane microvesicles as actors in the establishment of a favorable prostatic tumoral niche: a role for activated fibroblasts and CX3CL1-CX3CR1 axis. Cancer Res 2009; 69: 785–793.
Jung T, Castellana D, Klingbeil P, Cuesta Hernandez I, Vitacolonna M, Orlicky DJ et al. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia 2009; 11: 1093–1105.
Narumiya S, Tanji M, Ishizaki T . Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev 2009; 28: 65–76.
Li B, Antonyak MA, Druso JE, Cheng L, Nikitin AY, Cerione RA . EGF potentiated oncogenesis requires a tissue transglutaminase-dependent signaling pathway leading to Src activation. Proc Natl Acad Sci USA 2010; 107: 1408–1413.
Yuan L, Siegel M, Choi K, Khosla C, Miller CR, Jackson EN et al. Transglutaminase 2 inhibitor, KCC009, disrupts fibronectin assembly in the extracellular matrix and sensitizes orthotopic glioblastomas to chemotherapy. Oncogene 2007; 26: 2563–2573.
Teis D, Saksena S, Emr SD . SnapShot: the ESCRT machinery. Cell 2009; 137: 182.
Heasman SJ, Ridley AJ . Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol 2008; 9: 690–701.
Roberts PJ, Der CJ . Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007; 26: 3291–3310.
Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G et al. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol 2009; 19: 1875–1885.
Narumiya S, Ishizaki T, Uehata M . Use and properties of ROCK-specific inhibitor Y-27632. Methods Enzymol 2000; 325: 273–284.
Loirand G, Guerin P, Pacaud P . Rho kinases in cardiovascular physiology and pathophysiology. Circ Res 2006; 98: 322–334.
Kawano Y, Fukata Y, Oshiro N, Amano M, Nakamura T, Ito M et al. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol 1999; 147: 1023–1038.
Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 1999; 285: 895–898.
Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature 1998; 393: 809–812.
Petrache I, Birukov K, Zaiman AL, Crow MT, Deng H, Wadgaonkar R et al. Caspase-dependent cleavage of myosin light chain kinase (MLCK) is involved in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. FASEB J 2003; 17: 407–416.
Mbeunkui F, Johann Jr DJ . Cancer and the tumor microenvironment: a review of an essential relationship. Cancer Chemother Pharmacol 2009; 63: 571–582.
Davila M, Frost AR, Grizzle WE, Chakrabarti R . LIM kinase 1 is essential for the invasive growth of prostate epithelial cells: implications in prostate cancer. J Biol Chem 2003; 278: 36868–36875.
Davila M, Jhala D, Ghosh D, Grizzle WE, Chakrabarti R . Expression of LIM kinase 1 is associated with reversible G1/S phase arrest, chromosomal instability and prostate cancer. Mol Cancer 2007; 6: 40.
Yoshioka K, Foletta V, Bernard O, Itoh K . A role for LIM kinase in cancer invasion. Proc Natl Acad Sci USA 2003; 100: 7247–7252.
Bagheri-Yarmand R, Mazumdar A, Satin AA, Kumar R . LIM kinase 1 increases tumor metastasis of human breast cancer cells via regulation of the urokinase-type plasminogen activator system. Int J Cancer 2006; 118: 2703–2710.
Clark EA, Golub TR, Lander ES, Hynes RO . Genomic analysis of metastasis reveals an essential role for RhoC. Nature 2000; 406: 532–535.
Vega FM, Ridley AJ . Rho GTPases in cancer cell biology. FEBS Lett 2008; 582: 2093–2101.
Micuda S, Rösel D, Ryska A, Brábek J . ROCK inhibitors as emerging therapeutic candidates for sarcomas. Curr Cancer Drug Targets 2010; 10: 127–134.
Fackler OT, Grosse R . Cell motility through plasma membrane blebbing. J Cell Biol 2008; 181: 879–884.
Di Vizio D, Kim J, Hager MH, Morello M, Yang W, Lafargue CJ et al. Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res 2009; 69: 5601–5609.
Charras GT, Hu CK, Coughlin M, Mitchison TJ . Reassembly of contractile actin cortex in cell blebs. J Cell Biol 2006; 175: 477–490.
Acknowledgements
We thank Cindy Westmiller for her secretarial assistance and the NIH and the Komen Foundation for funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Li, B., Antonyak, M., Zhang, J. et al. RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene 31, 4740–4749 (2012). https://doi.org/10.1038/onc.2011.636
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.636
Keywords
This article is cited by
-
Extracellular vesicle-based liquid biopsy biomarkers and their application in precision immuno-oncology
Biomarker Research (2023)
-
Relevance of multilamellar and multicompartmental vesicles in biological fluids: understanding the significance of proportional variations and disease correlation
Biomarker Research (2023)
-
Exosomal cargos-mediated metabolic reprogramming in tumor microenvironment
Journal of Experimental & Clinical Cancer Research (2023)
-
Extracellular vesicle-associated tyrosine kinase-like orphan receptors ROR1 and ROR2 promote breast cancer progression
Cell Communication and Signaling (2023)
-
Context-specific regulation of extracellular vesicle biogenesis and cargo selection
Nature Reviews Molecular Cell Biology (2023)