Abstract
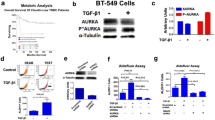
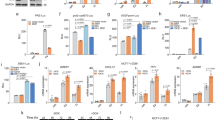
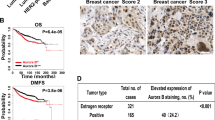
In this study, we demonstrate that constitutive activation of Raf-1 oncogenic signaling induces stabilization and accumulation of Aurora-A mitotic kinase that ultimately drives the transition from an epithelial to a highly invasive mesenchymal phenotype in estrogen receptor α-positive (ERα+) breast cancer cells. The transition from an epithelial- to a mesenchymal-like phenotype was characterized by reduced expression of ERα, HER-2/Neu overexpression and loss of CD24 surface receptor (CD24−/low). Importantly, expression of key epithelial-to-mesenchymal transition (EMT) markers and upregulation of the stemness gene SOX2 was linked to acquisition of stem cell-like properties such as the ability to form mammospheres in vitro and tumor self-renewal in vivo. Moreover, aberrant Aurora-A kinase activity induced phosphorylation and nuclear translocation of SMAD5, indicating a novel interplay between Aurora-A and SMAD5 signaling pathways in the development of EMT, stemness and ultimately tumor progression. Importantly, pharmacological and molecular inhibition of Aurora-A kinase activity restored a CD24+ epithelial phenotype that was coupled to ERα expression, downregulation of HER-2/Neu, inhibition of EMT and impaired self-renewal ability, resulting in the suppression of distant metastases. Taken together, our findings show for the first time the causal role of Aurora-A kinase in the activation of EMT pathway responsible for the development of distant metastases in ERα+ breast cancer cells. Moreover, this study has important translational implications because it highlights the mitotic kinase Aurora-A as a novel promising therapeutic target to selectively eliminate highly invasive cancer cells and improve the disease-free and overall survival of ERα+ breast cancer patients resistant to conventional endocrine therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Basaran G, Devrim C, Caglar HB, Gulluoglu B, Kaya H, Seber S et al. Clinical outcome of breast cancer patients with N3a (>/=10 positive lymph nodes) disease: has it changed over years? Med Oncol 2010; 28: 726–732.
Chang H, Rha SY, Jeung HC, Im CK, Ahn JB, Kwon WS et al. Association of the ABCB1 gene polymorphisms 2677G>T/A and 3435C>T with clinical outcomes of paclitaxel monotherapy in metastatic breast cancer patients. Ann Oncol 2009; 20: 272–277.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133: 704–715.
Hwang-Verslues WW, Kuo WH, Chang PH, Pan CC, Wang HH, Tsai ST et al. Multiple lineages of human breast cancer stem/progenitor cells identified by profiling with stem cell markers. PLoS One 2009; 4: e8377.
D’Assoro AB, Leontovich A, Amato A, Ayers-Ringler JR, Quatraro C, Hafner K et al. Abrogation of p53 function leads to metastatic transcriptome networks that typify tumor progression in human breast cancer xenografts. Int J Oncol 2010; 37: 1167–1176.
Guarino M, Rubino B, Ballabio G . The role of epithelial–mesenchymal transition in cancer pathology. Pathology 2007; 39: 305–318.
Beavon IR . The E-cadherin–catenin complex in tumour metastasis: structure, function and regulation. Eur J Cancer 2000; 36: 1607–1620.
Strathdee G . Epigenetic versus genetic alterations in the inactivation of E-cadherin. Semin Cancer Biol 2002; 12: 373–379.
Dean M, Fojo T, Bates S . Tumour stem cells and drug resistance. Nat Rev Cancer 2005; 5: 275–284.
Turley EA, Veiseh M, Radisky DC, Bissell MJ . Mechanisms of disease: epithelial–mesenchymal transition—does cellular plasticity fuel neoplastic progression? Nat Clin Pract Oncol 2008; 5: 280–290.
Wicha MS, Liu S, Dontu G . Cancer stem cells: an old idea—a paradigm shift. Cancer Res 2006; 66: 1883–1890 discussion 95–96.
Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M, Brauch H . Prevalence of CD44+/CD24−/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res 2005; 11: 1154–1159.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF . Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003; 100: 3983–3988.
Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lovgren K et al. The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res 2008; 10: R53.
Giatromanolaki A, Sivridis E, Fiska A, Koukourakis MI . The CD44+/CD24− phenotype relates to ‘triple-negative’ state and unfavorable prognosis in breast cancer patients. Med Oncol 2011; 28: 745–752.
Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J et al. Molecular definition of breast tumor heterogeneity. Cancer Cell 2007; 11: 259–273.
Wendt MK, Smith JA, Schiemann WP . Transforming growth factor-beta-induced epithelial–mesenchymal transition facilitates epidermal growth factor-dependent breast cancer progression. Oncogene 2010; 29: 6485–6498.
Murohashi M, Hinohara K, Kuroda M, Isagawa T, Tsuji S, Kobayashi S et al. Gene set enrichment analysis provides insight into novel signalling pathways in breast cancer stem cells. Br J Cancer 2009; 102: 206–212.
Eralp Y, Derin D, Ozluk Y, Yavuz E, Guney N, Saip P et al. MAPK overexpression is associated with anthracycline resistance and increased risk for recurrence in patients with triple-negative breast cancer. Ann Oncol 2008; 19: 669–674.
Jin W, Wu L, Liang K, Liu B, Lu Y, Fan Z . Roles of the PI-3K and MEK pathways in Ras-mediated chemoresistance in breast cancer cells. Br J Cancer 2003; 89: 185–191.
Ghayad SE, Vendrell JA, Larbi SB, Dumontet C, Bieche I, Cohen PA . Endocrine resistance associated with activated ErbB system in breast cancer cells is reversed by inhibiting MAPK or PI3K/Akt signaling pathways. Int J Cancer 2010; 126: 545–562.
Whyte J, Bergin O, Bianchi A, McNally S, Martin F . Key signalling nodes in mammary gland development and cancer. Mitogen-activated protein kinase signalling in experimental models of breast cancer progression and in mammary gland development. Breast Cancer Res 2009; 11: 209.
Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P et al. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci USA 1998; 95: 14821–14826.
Marampon F, Ciccarelli C, Zani BM . Down-regulation of c-Myc following MEK/ERK inhibition halts the expression of malignant phenotype in rhabdomyosarcoma and in non muscle-derived human tumors. Mol Cancer 2006; 5: 31.
Kim ES, Kim MS, Moon A . Transforming growth factor (TGF)-beta in conjunction with H-ras activation promotes malignant progression of MCF10A breast epithelial cells. Cytokine 2005; 29: 84–91.
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989; 244: 707–712.
Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS . ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol 2001; 3: 785–792.
Aranda V, Haire T, Nolan ME, Calarco JP, Rosenberg AZ, Fawcett JP et al. Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat Cell Biol 2006; 8: 1235–1245.
Kim IY, Yong HY, Kang KW, Moon A . Overexpression of ErbB2 induces invasion of MCF10A human breast epithelial cells via MMP-9. Cancer Lett 2009; 275: 227–233.
Korkaya H, Paulson A, Iovino F, Wicha MS . HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene 2008; 27: 6120–6130.
Roesler R, Cornelio DB, Abujamra AL, Schwartsmann G . HER2 as a cancer stem-cell target. Lancet Oncol 2010; 11: 225–226.
Korkaya H, Wicha MS . Cancer stem cells: nature versus nurture. Nat Cell Biol 2010; 12: 419–421.
Basto R, Brunk K, Vinadogrova T, Peel N, Franz A, Khodjakov A et al. Centrosome amplification can initiate tumorigenesis in flies. Cell 2008; 133: 1032–1042.
Thiery JP, Acloque H, Huang RY, Nieto MA . Epithelial–mesenchymal transitions in development and disease. Cell 2009; 139: 871–890.
Kerkhoff E, Fedorov LM, Siefken R, Walter AO, Papadopoulos T, Rapp UR . Lung-targeted expression of the c-Raf-1 kinase in transgenic mice exposes a novel oncogenic character of the wild-type protein. Cell Growth Differ 2000; 11: 185–190.
Chang CJ, Yang JY, Xia W, Chen CT, Xie X, Chao CH et al. EZH2 promotes expansion of breast tumor initiating cells through activation of RAF1-beta-catenin signaling. Cancer Cell 2011; 19: 86–100.
Yu D, Hung MC . Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene 2000; 19: 6115–6121.
Yu D, Hung MC . Role of erbB2 in breast cancer chemosensitivity. BioEssays 2000; 22: 673–680.
Lu J, Guo H, Treekitkarnmongkol W, Li P, Zhang J, Shi B et al. 14-3-3zeta Cooperates with ErbB2 to promote ductal carcinoma in situ progression to invasive breast cancer by inducing epithelial–mesenchymal transition. Cancer Cell 2009; 16: 195–207.
Yu X, Minter-Dykhouse K, Malureanu L, Zhao WM, Zhang D, Merkle CJ et al. Chfr is required for tumor suppression and Aurora A regulation. Nat Genet 2005; 37: 401–406.
Davis JM, Navolanic PM, Weinstein-Oppenheimer CR, Steelman LS, Hu W, Konopleva M et al. Raf-1 and Bcl-2 induce distinct and common pathways that contribute to breast cancer drug resistance. Clin Cancer Res 2003; 9: 1161–1170.
D'Assoro AB, Barrett SL, Folk C, Negron VC, Boeneman K, Busby R et al. Amplified centrosomes in breast cancer: a potential indicator of tumor aggressiveness. Breast Cancer Res Treat 2002; 75: 25–34.
D'Assoro AB, Busby R, Suino K, Delva E, Almodovar-Mercado GJ, Johnson H et al. Genotoxic stress leads to centrosome amplification in breast cancer cell lines that have an inactive G1/S cell cycle checkpoint.. Oncogene 2004; 23: 4068–4075.
D'Assoro AB, Busby R, Acu ID, Quatraro C, Reinholz MM, Farrugia DJ et al. Impaired p53 function leads to centrosome amplification, acquired ERalpha phenotypic heterogeneity and distant metastases in breast cancer MCF-7 xenografts. Oncogene 2008; 27: 3901–3911.
Acknowledgements
This study was supported by NCI CA72836 to JLS, USAMRMC BC022276 and Intramural RECDA Award to ABD, the Italian Association for Cancer Research (AIRC) to AA, the Mayo Clinic Breast Cancer Specialized Program of Research Excellence NIH CA116201 to JI and the Mayo Clinic School of Medicine. This study was also supported by the Atwater Foundation. We also wish to acknowledge the Cytogenetic Shared Resource and TACMA core facilities of the Mayo Clinic Comprehensive Cancer Center for performing SKY, routine cytogenetic and immunohistochemistry analysis and assisting us with the interpretation of the results.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
D'Assoro, A., liu, T., Quatraro, C. et al. The mitotic kinase Aurora-A promotes distant metastases by inducing epithelial-to-mesenchymal transition in ERα+ breast cancer cells. Oncogene 33, 599–610 (2014). https://doi.org/10.1038/onc.2012.628
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.628
Keywords
This article is cited by
-
LncRNA TIALD contributes to hepatocellular carcinoma metastasis via inducing AURKA lysosomal degradation
Cell Death Discovery (2023)
-
Cyclers’ kinases in cell division: from molecules to cancer therapy
Cell Death & Differentiation (2023)
-
E3 ubiquitin ligases in cancer stem cells: key regulators of cancer hallmarks and novel therapeutic opportunities
Cellular Oncology (2023)
-
Aurora kinase a promotes the progression of papillary thyroid carcinoma by activating the mTORC2-AKT signalling pathway
Cell & Bioscience (2022)
-
Mitotic protein kinase-driven crosstalk of machineries for mitosis and metastasis
Experimental & Molecular Medicine (2022)