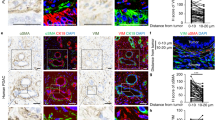
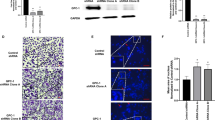
Abstract
Activation of myofibroblast rich stroma is a rate-limiting step essential for cancer progression. The responsible factors are not fully understood, but TGFβ1 is probably critical. A proportion of TGFβ1 is associated with extracellular nano-vesicles termed exosomes, secreted by carcinoma cells, and the relative importance of soluble and vesicular TGFβ in stromal activation is presented. Prostate cancer exosomes triggered TGFβ1-dependent fibroblast differentiation, to a distinctive myofibroblast phenotype resembling stromal cells isolated from cancerous prostate tissue; supporting angiogenesis in vitro and accelerating tumour growth in vivo. Myofibroblasts generated using soluble TGFβ1 were not pro-angiogenic or tumour-promoting. Cleaving heparan sulphate side chains from the exosome surface had no impact on TGFβ levels yet attenuated SMAD-dependent signalling and myofibroblastic differentiation. Eliminating exosomes from the cancer cell secretome, targeting Rab27a, abolished differentiation and lead to failure in stroma-assisted tumour growth in vivo. Exosomal TGFβ1 is therefore required for the formation of tumour-promoting stroma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Yanagisawa N, Li R, Rowley D, Liu H, Kadmon D, Miles BJ et al. Stromogenic prostatic carcinoma pattern (carcinomas with reactive stromal grade 3) in needle biopsies predicts biochemical recurrence-free survival in patients after radical prostatectomy. Hum Pathol 2007; 38: 1611–1620.
Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci 2010; 107: 20009–20014.
Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR . Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 1999; 59: 5002–5011.
Tuxhorn JA, McAlhany SJ, Yang F, Dang TD, Rowley DR . Inhibition of transforming growth factor-β activity decreases angiogenesis in a human prostate cancer-reactive stroma xenograft model. Cancer Res 2002; 62: 6021–6025.
Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR . Reactive stroma in human prostate cancer. Clin Cancer Res 2002; 8: 2912–2923.
Farmer P, Bonnefoi H, Anderle P, Cameron D, Wirapati P, Becette V et al. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med 2009; 15: 68–74.
Moorman AM, Vink R, Heijmans HJ, van der Palen J, Kouwenhoven EA . The prognostic value of tumour-stroma ratio in triple-negative breast cancer. Eur J Surg Oncol 2012; 38: 307–313.
Mesker WE, Liefers G-J, Junggeburt JMC, van Pelt GW, Alberici P, Kuppen PJK et al. Presence of a high amount of stroma and downregulation of SMAD4 predict for worse survival for stage I–II colon cancer patients. Cell Oncol 2009; 31: 169–178.
Verona EV, Elkahloun AG, Yang J, Bandyopadhyay A, Yeh I-T, Sun L-Z . Transforming growth factor-β signaling in prostate stromal cells supports prostate carcinoma growth by up-regulating stromal genes related to tissue remodeling. Cancer Res 2007; 67: 5737–5746.
Renzoni E, Abraham D, Howat S, Shi-Wen X, Sestini P, Bou-Gharios G et al. Gene expression profiling reveals novel TGFbeta targets in adult lung fibroblasts. Respir Res 2004; 5: 24.
Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Path 2012; 180: 1340–1355.
Webber J, Steadman R, Tabi Z, Mason MD, Clayton A . Cancer exosomes activate fibroblast to myofibroblast differentiation in a TGFβ dependent manner. Cancer Res 2010; 70: 9621–9630.
Gu J, Qian H, Shen L, Zhang X, Zhu W, Huang L et al. Gastric cancer exosomes trigger differentiation of umbilical cord derived mesenchymal stem cells to carcinoma-associated fibroblasts through TGF-β/Smad pathway. PLoS One 2012; 7: e52465.
Raposo G, Nijman HW, Stoorvogel W, Leijendekker R, Harding CV, Melief CJM et al. B Lymphocytes secrete antigen-presenting vesicles. J Exp Med 1996; 183: 1161–1172.
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008; 319: 1244–1247.
Simpson RJ, Lim JW, Moritz RL, Mathivanan S . Exosomes: proteomic insights and diagnostic potential. Exp Rev Proteomics 2009; 6: 267–283.
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO . Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9: 654–659.
Thery C, Ostrowski M, Segura E . Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009; 9: 581–593.
Junji W, Hideya O, Hiroyuki S, Akio Y, Shuntaro N, Takashi M et al. Surface-bound TGF-β on effusion-derived exosomes participates in maintenance of number and suppressive function of regulatory T-Cells in malignant effusions. Anticancer Res 2010; 30: 3747–3757.
Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z . Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol 2008; 180: 7249–7258.
Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z . Human tumour-derived exosomes selectively impair lymphocyte responses to Interleukin-2. Cancer Res 2007; 67: 7458–7466.
Hood JL, San RS, Wickline SA . Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res 2011; 71: 3792–3801.
Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012; 18: 883–891.
Sheldon H, Heikamp E, Turley H, Dragovic R, Thomas P, Oon CE et al. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood 2010; 116: 2385–2394.
Gesierich S, Berezovskiy I, Ryschich E, Zoller M . Systemic induction of the angiogenesis switch by the tetraspanin D6.1A/CO-029. Cancer Res 2006; 66: 7083–7094.
Hood JL, Pan H, Lanza GM, Wickline SA . Paracrine induction of endothelium by tumor exosomes. Lab Invest 2009; 89: 1317–1328.
Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M et al. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res 2010; 70: 1668–1678.
Richman PI, Tilly R, Jass JR, Bodmer WF . Colonic pericrypt sheath cells: characterisation of cell type with new monoclonal antibody. J Clin Pathol 1987; 40: 593–600.
Hetheridge C, Mavria G, Mellor H . Uses of the in vitro endothelial-fibroblast organotypic co-culture assay in angiogenesis research. Biochem Soc Trans 2011; 39: 1597–1600.
Lories V, Cassiman JJ, Van den Berghe H, David G . Multiple distinct membrane heparan sulfate proteoglycans in human lung fibroblasts. J Biol Chem 1989; 264: 7009–7016.
Hashizume H, Falcon BL, Kuroda T, Baluk P, Coxon A, Yu D et al. Complementary actions of inhibitors of angiopoietin-2 and VEGF on tumor angiogenesis and growth. Cancer Res 2010; 70: 2213–2223.
Jones JL, Anderson JM, Phuah CL, Fox EJ, Selmaj K, Margolin D et al. Improvement in disability after alemtuzumab treatment of multiple sclerosis is associated with neuroprotective autoimmunity. Brain 2010; 133 (Pt 8): 2232–2247.
Stefansson S, McMahon GA, Petitclerc E, Lawrence DA . Plasminogen activator inhibitor-1 in tumor growth, angiogenesis and vascular remodeling. Curr Pharm Des 2003; 9: 1545–1564.
Ma PC, Jagadeeswaran R, Jagadeesh S, Tretiakova MS, Nallasura V, Fox EA et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res 2005; 65: 1479–1488.
Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 2010; 12: 19–30.
Massague J . Receptors for the TGF-beta family. Cell 1992; 69: 1067–1070.
Mitchell JP, Court J, Mason MD, Tabi Z, Clayton A . Increased exosome production from tumour cell cultures using the Integra CELLine Culture System. J Immunol Methods 2008; 335: 98–105.
Lamparski H, Metha-Damani A, Yao J, Patel S, Hsu D, Ruegg C et al. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods 2002; 270: 211–226.
Welton JL, Khanna S, Giles PJ, Brennan P, Brewis IA, Staffurth J et al. Proteomic analysis of bladder cancer exosomes. Mol Cell Proteomics 2010; 6: 1324–1338.
Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z . Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol 2011; 187: 676–683.
Zuker M . Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 2003; 31: 3406–3415.
Jiang WG, Davies G, Martin TA, Kynaston H, Mason MD, Fodstad O . Com-1/p8 acts as a putative tumour suppressor in prostate cancer. Int J Mol Med 2006; 18: 981–986.
Webber J, Meran S, Steadman R, Phillips A . Hyaluronan orchestrates transforming growth factor-beta1-dependent maintenance of myofibroblast phenotype. J Biol Chem 2009; 284: 9083–9092.
Acknowledgements
This work was supported by a Cancer Research Wales programme grant awarded to AC, ZT, WGJ and MDM. Confocal microscopy studies were supported by a CR-UK studentship awarded to JPW and ATJ. In vivo studies were supported by a CR-UK development grant to AC and JPW. Drs Jane Lane and Tracey Martin are acknowledged for their help with the in vivo work. Dr H Sheldon and Professor A Harris, Oxford University, UK assisted with establishing in vitro angiogenesis assays.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Webber, J., Spary, L., Sanders, A. et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene 34, 290–302 (2015). https://doi.org/10.1038/onc.2013.560
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.560
Keywords
This article is cited by
-
Prostate cancer-derived small extracellular vesicle proteins: the hope in diagnosis, prognosis, and therapeutics
Journal of Nanobiotechnology (2023)
-
Machine learning-based analysis of cancer cell-derived vesicular proteins revealed significant tumor-specificity and predictive potential of extracellular vesicles for cell invasion and proliferation – A meta-analysis
Cell Communication and Signaling (2023)
-
Cutaneous squamous cell carcinoma-derived extracellular vesicles exert an oncogenic role by activating cancer-associated fibroblasts
Cell Death Discovery (2023)
-
Comprehensive insight into endothelial progenitor cell-derived extracellular vesicles as a promising candidate for disease treatment
Stem Cell Research & Therapy (2022)
-
Intercellular crosstalk between cancer cells and cancer-associated fibroblasts via extracellular vesicles
Cancer Cell International (2022)