Abstract
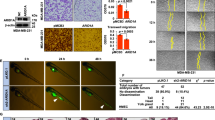
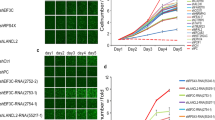
We recently demonstrated that expression of ADP-ribosylation factor (ARF)-like 4c (Arl4c) induced by a combination of Wnt/β-catenin and epidermal growth factor/Ras signaling in normal epithelial cells grown in three-dimensional culture promotes cellular migration and proliferation, resulting in formation of tube-like structures, suggesting the involvement of Arl4c in epithelial morphogenesis. It is conceivable that there could be a common mechanism between epithelial morphogenesis and carcinogenesis. Therefore the current study was conducted to investigate whether Arl4c might be involved in tumorigenesis. Immunohistochemical analyses of tissue specimens obtained from colorectal and lung cancer patients revealed that Arl4c was not observed in non-tumor regions but was strongly expressed at high frequencies in tumor lesions. Inhibition of Wnt/β-catenin or Ras/mitogen-activated protein kinase signaling reduced Arl4c mRNA levels in HCT116 colorectal cancer cells and A549 lung cancer cells. Knockdown of Arl4c inhibited Rac activity and also prevented nuclear localization of yes-associated protein (YAP)/transcriptional co-activator with PDZ-binding motif (TAZ) in these cancer cells. Arl4c-depleted cancer cells consistently showed decreased migration, invasion and proliferation capabilities both in vitro and in vivo. Furthermore, direct injection of Arl4c small interfering RNA (siRNA) into HCT116 cell-derived tumors (in vivo treatment with siRNA) inhibited tumor growth in immunodeficient mice. These results suggest that Arl4c is involved in tumorigenesis and might represent a novel therapeutic target for suppressing proliferation and invasion of colorectal and lung cancer cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
O'Brien LE, Zegers MM, Mostov KE . Opinion: Building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol 2002; 3: 531–537.
Debnath J, Brugge JS . Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer 2005; 5: 675–688.
Matsumoto S, Fujii S, Sato A, Ibuka S, Kagawa Y, Ishii M et al. A combination of Wnt and growth factor signaling induces Arl4c expression to form epithelial tubular structures. EMBO J 2014; 33: 702–718.
Burd CG, Strochlic TI, Gangi Setty SR . Arf-like GTPases: not so Arf-like after all. Trends Cell Biol 2004; 14: 687–694.
Pasqualato S, Renault L, Cherfils J . Arf, Arl, Arp and Sar proteins: a family of GTP-binding proteins with a structural device for 'front-back' communication. EMBO Rep 2002; 3: 1035–1041.
Jacobs S, Schilf C, Fliegert F, Koling S, Weber Y, Schurmann A et al. ADP-ribosylation factor (ARF)-like 4, 6, and 7 represent a subgroup of the ARF family characterization by rapid nucleotide exchange and a nuclear localization signal. FEBS Lett 1999; 456: 384–388.
Engel T, Lueken A, Bode G, Hobohm U, Lorkowski S, Schlueter B et al. ADP-ribosylation factor (ARF)-like 7 (ARL7) is induced by cholesterol loading and participates in apolipoprotein AI-dependent cholesterol export. FEBS Lett 2004; 566: 241–246.
Wei SM, Xie CG, Abe Y, Cai JT . ADP-ribosylation factor like 7 (ARL7) interacts with α-tubulin and modulates intracellular vesicular transport. Biochem Biophys Res Commun 2009; 384: 352–356.
McCormick F . Signalling networks that cause cancer. Trends Cell Biol 1999; 9: M53–M56.
Polakis P . The many ways of Wnt in cancer. Curr Opin Genet Dev 2007; 17: 45–51.
Clevers H, Nusse R . Wnt/β-catenin signaling and disease. Cell 2012; 149: 1192–1205.
Kikuchi A, Yamamoto H, Sato A, Matsumoto S . New insights into the mechanism of wnt signaling pathway activation. Int Rev Cell Mol Biol 2011; 291: 21–71.
Sekido Y, Fong KM, Minna JD . Molecular genetics of lung cancer. Annu Rev Med 2003; 54: 73–87.
Mori M, Rao SK, Popper HH, Cagle PT, Fraire AE . Atypical adenomatous hyperplasia of the lung: a probable forerunner in the development of adenocarcinoma of the lung. Mod Pathol 2001; 14: 72–84.
Gayet J, Zhou XP, Duval A, Rolland S, Hoang JM, Cottu P et al. Extensive characterization of genetic alterations in a series of human colorectal cancer cell lines. Oncogene 2001; 20: 5025–5032.
Yang J, Zhang W, Evans PM, Chen X, He X, Liu C . Adenomatous polyposis coli (APC) differentially regulates β-catenin phosphorylation and ubiquitination in colon cancer cells. J Biol Chem 2006; 281: 17751–17757.
She QB, Halilovic E, Ye Q, Zhen W, Shirasawa S, Sasazuki T et al. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell 2010; 18: 39–51.
Krypuy M, Newnham GM, Thomas DM, Conron M, Dobrovic A . High resolution melting analysis for the rapid and sensitive detection of mutations in clinical samples: KRAS codon 12 and 13 mutations in non-small cell lung cancer. BMC Cancer 2006; 6: 295.
Sunaga N, Shames DS, Girard L, Peyton M, Larsen JE, Imai H et al. Knockdown of oncogenic KRAS in non-small cell lung cancers suppresses tumor growth and sensitizes tumor cells to targeted therapy. Mol Cancer Ther 2011; 10: 336–346.
Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol 2009; 5: 100–107.
Hashimoto S, Onodera Y, Hashimoto A, Tanaka M, Hamaguchi M, Yamada A et al. Requirement for Arf6 in breast cancer invasive activities. Proc Natl Acad Sci USA 2004; 101: 6647–6652.
Salhia B, Rutten F, Nakada M, Beaudry C, Berens M, Kwan A et al. Inhibition of Rho-kinase affects astrocytoma morphology, motility, and invasion through activation of Rac1. Cancer Res 2005; 65: 8792–8800.
Kagawa Y, Matsumoto S, Kamioka Y, Mimori K, Naito Y, Ishii T et al. Cell cycle-dependent Rho GTPase activity dynamically regulates cancer cell motility and invasion in vivo. PLoS ONE 2013; 8: e83629.
Shitashige M, Satow R, Jigami T, Aoki K, Honda K, Shibata T et al. Traf2- and Nck-interacting kinase is essential for Wnt signaling and colorectal cancer growth. Cancer Res 2010; 70: 5024–5033.
Polakis P, McCormick F . Structural requirements for the interaction of p21ras with GAP, exchange factors, and its biological effector target. J Biol Chem 1993; 268: 9157–9160.
Hofmann I, Thompson A, Sanderson CM, Munro S . The Arl4 family of small G proteins can recruit the cytohesin Arf6 exchange factors to the plasma membrane. Curr Biol 2007; 17: 711–716.
Li CC, Chiang TC, Wu TS, Pacheco-Rodriguez G, Moss J, Lee FJ . ARL4D recruits cytohesin-2/ARNO to modulate actin remodeling. Mol Biol Cell 2007; 18: 4420–4437.
Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 2012; 493: 106–110.
Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell 2014; 25: 166–180.
Feinberg AP . Phenotypic plasticity and the epigenetics of human disease. Nature 2007; 447: 433–440.
Ji H, Ehrlich LI, Seita J, Murakami P, Doi A, Lindau P et al. Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature 2010; 467: 338–342.
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–2388.
Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M et al. A small molecule inhibitor of β-catenin/CREB-binding protein transcription [corrected]. Proc Natl Acad Sci USA 2004; 101: 12682–12687.
Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 2009; 461: 614–620.
Nieto MA . Epithelial plasticity: a common theme in embryonic and cancer cells. Science 2013; 342: 1234850.
Dorsett Y, Tuschl T . siRNAs: applications in functional genomics and potential as therapeutics. Nat Rev Drug Discov 2004; 3: 318–329.
Ikeda J, Oda T, Inoue M, Uekita T, Sakai R, Okumura M et al. Expression of CUB domain containing protein (CDCP1) is correlated with prognosis and survival of patients with adenocarcinoma of lung. Cancer Sci 2009; 100: 429–433.
Wang L, Shi S, Guo Z, Zhang X, Han S, Yang A et al. Overexpression of YAP and TAZ is an independent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells. PLoS ONE 2013; 8: e65539.
Tsai HC, Li H, Van Neste L, Cai Y, Robert C, Rassool FV et al. Transient low doses of DNA-demethylating agents exert durable antitumor effects on hematological and epithelial tumor cells. Cancer Cell 2012; 21: 430–446.
Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H et al. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 2012; 22: 66–79.
Miyoshi H, Blomer U, Takahashi M, Gage FH, Verma IM . Development of a self-inactivating lentivirus vector. J Virol 1998; 72: 8150–8157.
Sato A, Yamamoto H, Sakane H, Koyama H, Kikuchi A . Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO J 2010; 29: 41–54.
Gon H, Fumoto K, Ku Y, Matsumoto S, Kikuchi A . Wnt5a signaling promotes apical and basolateral polarization of single epithelial cells. Mol Biol Cell 2013; 24: 3764–3774.
Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T et al. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res 2006; 66: 10439–10448.
Yamamoto H, Kitadai Y, Yamamoto H, Oue N, Ohdan H, Yasui W et al. Laminin γ2 mediates Wnt5a-induced invasion of gastric cancer cells. Gastroenterology 2009; 137: 242–252.
Matsumoto S, Fumoto K, Okamoto T, Kaibuchi K, Kikuchi A . Binding of APC and dishevelled mediates Wnt5a-regulated focal adhesion dynamics in migrating cells. EMBO J 2010; 29: 1192–1204.
Hanaki H, Yamamoto H, Sakane H, Matsumoto S, Ohdan H, Sato A et al. An anti-Wnt5a antibody suppresses metastasis of gastric cancer cells in vivo by inhibiting receptor-mediated endocytosis. Mol Cancer Ther 2012; 11: 298–307.
Acknowledgements
We thank Dr Y Shintani and Dr K Shojima for valuable discussions regarding Arl4c expression and their technical assistance. We also thank Dr T Kobayashi, Dr W Yasui and Dr K Matsumoto for donating cells. This work was supported by Grants-in-Aid for Scientific Research to AK (2013–2014) (No. 25250018), SF (2014) (No. 26861547) and SM (2013–2014) (No. 25860211) and for Scientific Research on Innovative Areas to AK (2011–2014) (No. 23112004) from the Ministry of Education, Science and Culture of Japan and by grants from the Uehara Memorial Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
SF, SM and AK have received research funds from Shionogi and Co., Ltd. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Fujii, S., Matsumoto, S., Nojima, S. et al. Arl4c expression in colorectal and lung cancers promotes tumorigenesis and may represent a novel therapeutic target. Oncogene 34, 4834–4844 (2015). https://doi.org/10.1038/onc.2014.402
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.402
This article is cited by
-
ARL4C is associated with epithelial-to-mesenchymal transition in colorectal cancer
BMC Cancer (2023)
-
3D genome mapping identifies subgroup-specific chromosome conformations and tumor-dependency genes in ependymoma
Nature Communications (2023)
-
High miR203a-3p and miR-375 expression in the airways of smokers with and without COPD
Scientific Reports (2022)
-
A risk stratification model based on four novel biomarkers predicts prognosis for patients with renal cell carcinoma
World Journal of Surgical Oncology (2020)
-
The TRPV4-AKT axis promotes oral squamous cell carcinoma cell proliferation via CaMKII activation
Laboratory Investigation (2020)