Abstract
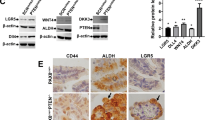
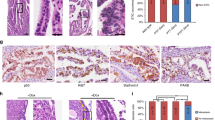
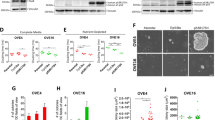
The signaling events involved in the onset of ovarian cancer from the fallopian tube epithelium (FTE) are crucial for early detection and treatment of the disease, but they remain poorly defined. Conditional homozygous knockout of PTEN mediated by PAX8-cre recombinase was sufficient to drive endometrioid and serous borderline ovarian carcinoma, providing the first model of FTE-derived borderline tumors. In addition, heterozygous PTEN deletion in the FTE resulted in hyperplasia, providing a model to study early events of human ovarian pathogenesis. To uncover the mechanism underlying the invasion of cancerous oviductal cells to the ovary, PTEN-deficient murine oviductal cells were developed and tagged with green fluorescent protein. Loss of PTEN increased cell migration, invasion, and upregulated WNT4, a key regulator of Müllerian duct development during embryogenesis. Further investigation revealed that WNT4 was required for increased migration and colonization of the ovary by PTEN-deficient oviductal cells in a β-catenin independent manner. Human tumor microarrays and ovarian cancer cells lines confirmed WNT4 expression in cancer and its role in migration. Together, these findings provide a novel model to study the mechanism of fallopian tube tumor initiation and invasion to the ovary mediated by loss of PTEN, which may help to define early events of human ovarian carcinogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
Kurman RJ, Shih IeM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–43.
Kuhn E, Kurman RJ, Vang R, Sehdev AS, Han G, Soslow R, et al. TP53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma--evidence supporting the clonal relationship of the two lesions. J Pathol. 2012;226:421–6.
Seidman JD, Horkayne-Szakaly I, Haiba M, Boice CR, Kurman RJ, Ronnett BM. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol. 2004;23:41–4.
Seidman JD, Zhao P, Yemelyanova A. “Primary peritoneal” high-grade serous carcinoma is very likely metastatic from serous tubal intraepithelial carcinoma: assessing the new paradigm of ovarian and pelvic serous carcinogenesis and its implications for screening for ovarian cancer. Gynecol Oncol. 2011;120:470–3.
Vang R, Shih IeM, Kurman RJ. Fallopian tube precursors of ovarian low- and high-grade serous neoplasms. Histopathology. 2013;62:44–58.
Przybycin CG, Kurman RJ, Ronnett BM, Shih le M, Vang R. Are all pelvic (nonuterine) serous carcinomas of tubal origin?. Am J Surg Pathol. 2010;34:1407–16.
Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–9.
Eddie SL, Quartuccio SM, Oh E, Moyle-Heyrman G, Lantvit DD, Wei JJ. et al. Tumorigenesis and peritoneal colonization from fallopian tube epithelium. Oncotarget. 2015;6:20500–12.
Shield-Artin KL, Bailey MJ, Oliva K, Liovic AK, Barker G, Dellios NL, et al. Identification of ovarian cancer-associated proteins in symptomatic women: a novel method for semi-quantitative plasma proteomics. Proteom Clin Appl. 2012;6:170–81.
Wu R, Zhai Y, Kuick R, Karnezis AN, Garcia P, Naseem A, et al. Impact of oviductal versus ovarian epithelial cell of origin on ovarian endometrioid carcinoma phenotype in the mouse. J Pathol. 2016;240:341–51.
Li J, Abushahin N, Pang S, Xiang L, Chambers SK, Fadare O, et al. Tubal origin of ‘ovarian’ low-grade serous carcinoma. Mod Pathol. 2011;24:1488–99.
Martins FC, Santiago I, Trinh A, Xian J, Guo A, Sayal K, et al. Combined image and genomic analysis of high-grade serous ovarian cancer reveals PTEN loss as a common driver event and prognostic classifier. Genome Biol. 2014;15:526.
Hanrahan AJ, Schultz N, Westfal ML, Sakr RA, Giri DD, Scarperi S, et al. Genomic complexity and AKT dependence in serous ovarian cancer. Cancer Discov. 2014;2:56–67.
Roh MH, Yassin Y, Miron A, Mehra KK, Mehrad M, Monte NM, et al. High-grade fimbrial-ovarian carcinomas are unified by alteredp53, PTEN and PAX2 expression. Mod Pathol. 2010;23:1316–24.
McDaniel AS, Stall JN, Hovelson DH, Cani AK, Liu CJ, Tomlins SA. et al. Next-generation sequencing of tubal intraepithelial carcinomas. JAMA Oncol. 2015;1:1128–32.
network Tcgar. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15.
Cho KR. Ovarian cancer update: lessons from morphology, molecules, and mice. Arch Pathol Lab Med. 2009;133:1775–81.
Sherman-Baust CA, Kuhn E, Valle BL, Shih Ie M, Kurman RJ, Wang TL, et al. A genetically engineered ovarian cancer mouse model based on fallopian tube transformation mimics human high-grade serous carcinoma development. J Pathol. 2014;233:228–37.
Kim J, Coffey DM, Creighton CJ, Yu Z, Hawkins SM, Matzuk MM. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc Natl Acad Sci USA. 2012;109:3921–6.
Zhai Y, Wu R, Kuick R, Sessine MS, Schulman S, Green M. et al. High-grade serous carcinomas arise in the mouse oviduct via defects linked to the human disease. J Pathol. 2017;243:16–25.
Coffman LG, Burgos-Ojeda D, Wu R, Cho K, Bai S, Buckanovich RJ. New models of hematogenous ovarian cancer metastasis demonstrate preferential spread to the ovary and a requirement for the ovary for abdominal dissemination. Transl Res. 2016;175:92–102.
Kispert A, Vainio S, McMahon AP. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development. 1998;125:4225–34.
Kobayashi A, Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet. 2003;4:969–80.
Kuchenbaecker KB, Ramus SJ, Tyrer J, Lee A, Shen HC, Beesley J, et al. Identification of six new susceptibility loci for invasive epithelial ovarian cancer. Nat Genet. 2015;47:164–71.
Ng A, Barker N. Ovary and fimbrial stem cells: biology, niche and cancer origins. Nat Rev Mol Cell Biol. 2015;16:625–38.
Prunskaite-Hyyrylainen R, Skovorodkin I, Xu Q, Miinalainen I, Shan J, Vainio SJ. Wnt4 coordinates directional cell migration and extension of the Mullerian duct essential for ontogenesis of the female reproductive tract. Hum Mol Genet. 2016;25:1059–73.
Cohen ED, Mariol MC, Wallace RM, Weyers J, Kamberov YG, Pradel J, et al. DWnt4 regulates cell movement and focal adhesion kinase during Drosophila ovarian morphogenesis. Dev Cell. 2002;2:437–48.
Endsley MP, Moyle-Heyrman G, Karthikeyan S, Lantvit DD, Davis DA, Wei JJ. et al. Spontaneous transformation of murine oviductal epithelial cells: a model system to investigate the onset of fallopian-derived tumors. Front Oncol. 2015;5:154
Modi DA, Tagare RD, Karthikeyan S, Russo A, Dean M, Davis DA. et al. PAX2 function, regulation and targeting in fallopian tube-derived high-grade serous ovarian cancer. Oncogene. 2016;36:3015–24.
Yang-Hartwich Y, Gurrea-Soteras M, Sumi N, Joo WD, Holmberg JC, Craveiro V, et al. Ovulation and extra-ovarian origin of ovarian cancer. Sci Rep. 2014;4:6116.
Fathalla MF. Incessant ovulation and ovarian cancer - a hypothesis re-visited. Facts Views Vis Obgyn. 2013;5:292–7.
Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–38.
Sood AK, Coffin JE, Schneider GB, Fletcher MS, DeYoung BR, Gruman LM, et al. Biological significance of focal adhesion kinase in ovarian cancer: role in migration and invasion. Am J Pathol. 2004;165:1087–95.
Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–96.
Ward KK, Tancioni I, Lawson C, Miller NL, Jean C, Chen XL, et al. Inhibition of focal adhesion kinase (FAK) activity prevents anchorage-independent ovarian carcinoma cell growth and tumor progression. Clin Exp Metastas-. 2013;30:579–94.
Perets R, Wyant GA, Muto KW, Bijron JG, Poole BB, Chin KT, et al. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell. 2013;24:751–65.
Mullany LK, Richards JS. Minireview: animal models and mechanisms of ovarian cancer development. Endocrinology. 2012;153:1585–92.
Zhang L, Ma T, Brozick J, Babalola K, Budiu R, Tseng G, et al. Effects of Kras activation and Pten deletion alone or in combination on MUC1 biology and epithelial-to-mesenchymal transition in ovarian cancer. Oncogene. 2016;35:5010–20.
Daikoku T, Hirota Y, Tranguch S, Joshi AR, DeMayo FJ, Lydon JP, et al. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res. 2008;68:5619–27.
Kurman RJ, Vang R, Junge J, Hannibal CG, Kjaer SK, Shih Ie M. Papillary tubal hyperplasia: the putative precursor of ovarian atypical proliferative (borderline) serous tumors, noninvasive implants, and endosalpingiosis. Am J Surg Pathol. 2011;35:1605–14.
Robey SS, Silva EG. Epithelial hyperplasia of the fallopian tube. Its association with serous borderline tumors of the ovary. Int J Gynecol Pathol. 1989;8:214–20.
Laury AR, Ning G, Quick CM, Bijron J, Parast MM, Betensky RA, et al. Fallopian tube correlates of ovarian serous borderline tumors. Am J Surg Pathol. 2011;35:1759–65.
Boone JD, Arend RC, Johnston BE, Cooper SJ, Gilchrist SA, Oelschlager DK, et al. Targeting the Wnt/beta-catenin pathway in primary ovarian cancer with the porcupine inhibitor WNT974. Lab Invest. 2016;96:249–59.
Karthikeyan S, Lantvit DD, Chae DH, Burdette JE. Cadherin-6 type 2, K-cadherin (CDH6) is regulated by mutant p53 in the fallopian tube but is not expressed in the ovarian surface. Oncotarget. 2016;7:69871–82.
Torban E, Dziarmaga A, Iglesias D, Chu LL, Vassilieva T, Little M, et al. PAX2 activates WNT4 expression during mammalian kidney development. J Biol Chem. 2006;281:12705–12.
Filippone MG, Di Palma T, Lucci V, Zannini M. Pax8 modulates the expression of Wnt4 that is necessary for the maintenance of the epithelial phenotype of thyroid cells. BMC Mol Biol. 2014;15:21.
King SM, Quartuccio SM, Vanderhyden BC, Burdette JE. Early transformative changes in normal ovarian surface epithelium induced by oxidative stress require Akt upregulation, DNA damage and epithelial-stromal interaction. Carcinogenesis. 2013;34:1125–33.
Haley J, Tomar S, Pulliam N, Xiong S, Perkins SM, Karpf AR, et al. Functional characterization of a panel of high-grade serous ovarian cancer cell lines as representative experimental models of the disease. Oncotarget. 2016;7:32810–20.
Bouchard M, Souabni A, Busslinger M. Tissue-specific expression of cre recombinase from the Pax8 locus. Genesis. 2004;38:105–9.
Quartuccio SM, Karthikeyan S, Eddie SL, Lantvit DD, Oh E, Modi DA, et al. Mutant p53 expression in fallopian tube epithelium drives cell migration. Int J Cancer. 2015;137:1528–38.
Sukerkar PA, MacRenaris KW, Townsend TR, Ahmed RA, Burdette JE, Meade TJ. Synthesis and biological evaluation of water-soluble progesterone-conjugated probes for magnetic resonance imaging of hormone related cancers. Bioconjug Chem. 2011;22:2304–16.
Hilliard TS, Modi DA, Burdette JE. Gonadotropins activate oncogenic pathways to enhance proliferation in normal mouse ovarian surface epithelium. Int J Mol Sci. 2013;14:4762–82.
Quartuccio SM, Lantvit DD, Bosland MC, Burdette JE. Conditional inactivation of p53 in mouse ovarian surface epithelium does not alter MIS driven Smad2-dominant negative epithelium-lined inclusion cysts or teratomas. PLoS One. 2013;8:e65067.
Acknowledgements
This work was supported by Tell Every Amazing Lady (T.E.A.L.) About Ovarian Cancer Louisa M. McGregor Ovarian Cancer Foundation, The American Cancer Society ACS RSG-12-230-01-TBG, the NIH UG3 ES029073-01 and the NIH T32 AT007533. We would also like to thank the Northwestern NUseq Core Facility and Dr. Matthew Schipma.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Russo, A., Czarnecki, A.A., Dean, M. et al. PTEN loss in the fallopian tube induces hyperplasia and ovarian tumor formation. Oncogene 37, 1976–1990 (2018). https://doi.org/10.1038/s41388-017-0097-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-017-0097-8
This article is cited by
-
MATN2 overexpression suppresses tumor growth in ovarian cancer via PTEN/PI3K/AKT pathway
Functional & Integrative Genomics (2024)
-
Tolfenamic acid inhibits ROS-generating oxidase Nox1-regulated p53 activity in intrastriatal injection of malonic acid rats
The Journal of Physiological Sciences (2022)
-
RAB4A GTPase regulates epithelial-to-mesenchymal transition by modulating RAC1 activation
Breast Cancer Research (2022)
-
MiR-135b improves proliferation and regulates chemotherapy resistance in ovarian cancer
Journal of Molecular Histology (2022)
-
Silencing PTEN in the fallopian tube promotes enrichment of cancer stem cell-like function through loss of PAX2
Cell Death & Disease (2021)